Chapter 18: Antipsychotics
2nd edition as of August 2022
Chapter Overview
The next class of psychotherapeutic drugs in this unit is antipsychotics. These are drugs used to treat schizophrenia, a mental illness that has been portrayed often in the news and other media, though not always accurately. To begin this chapter, we will examine the disorder itself and its symptoms, prevalence, and possible causes. The second half of the chapter will cover the family of drugs used to treat schizophrenia.
Chapter Outline
18.1. Overview of Schizophrenia
- 18.1.1. Symptoms and Prevalence
- 18.1.2. Causes of Schizophrenia
- 18.1.3. Dopamine Hypothesis of Schizophrenia
- 18.1.4. Glutamate Hypothesis of Schizophrenia
- 18.2.1. History and Overview
- 18.2.2. Administration and Pharmacokinetics
- 18.2.3. Mechanism of Action and Effects
- 18.2.4. Other Adverse Effects
- 18.2.5. Comparison of Antipsychotic Drugs
Chapter Learning Outcomes
- List and describe the symptoms of schizophrenia and state its prevalence.
- Discuss possible causes of schizophrenia.
- List and describe antipsychotic drugs used to treat schizophrenia.
18.1. Overview of Schizophrenia
Section Learning Objectives
- Define schizophrenia and its common characteristics.
- Describe possible causes of schizophrenia.
- Explain the two hypotheses for the neurochemical basis of schizophrenia.
Schizophrenia is a chronic and severe mental disorder characterized by abnormal behavior and loss of contact with reality. Although the term translates to “split mind,” it has nothing to do with split personalities. Instead, people with schizophrenia may experience hallucinations, delusions, or other symptoms that lead to a disconnect between the patient and the real world.
18.1.1. Symptoms and Prevalence
Before we begin this section, watch the video below to get an overview of schizophrenia. The video does a good job of explaining the types of symptoms and how the disorder is diagnosed. We will review the content covered in the video in the text below, but the video will make it much easier to understand.
Schizophrenia – causes, symptoms, diagnosis, treatment & pathology [8:14]
That was a lot of information. For now, let us focus on the symptoms of schizophrenia. As mentioned in the video, these can be grouped into positive, negative, and cognitive symptoms. Positive symptoms are psychotic behaviors added to normal behaviors, while negative symptoms are disruptions of normal behaviors and emotions. Below is a list of examples for each category of symptoms:
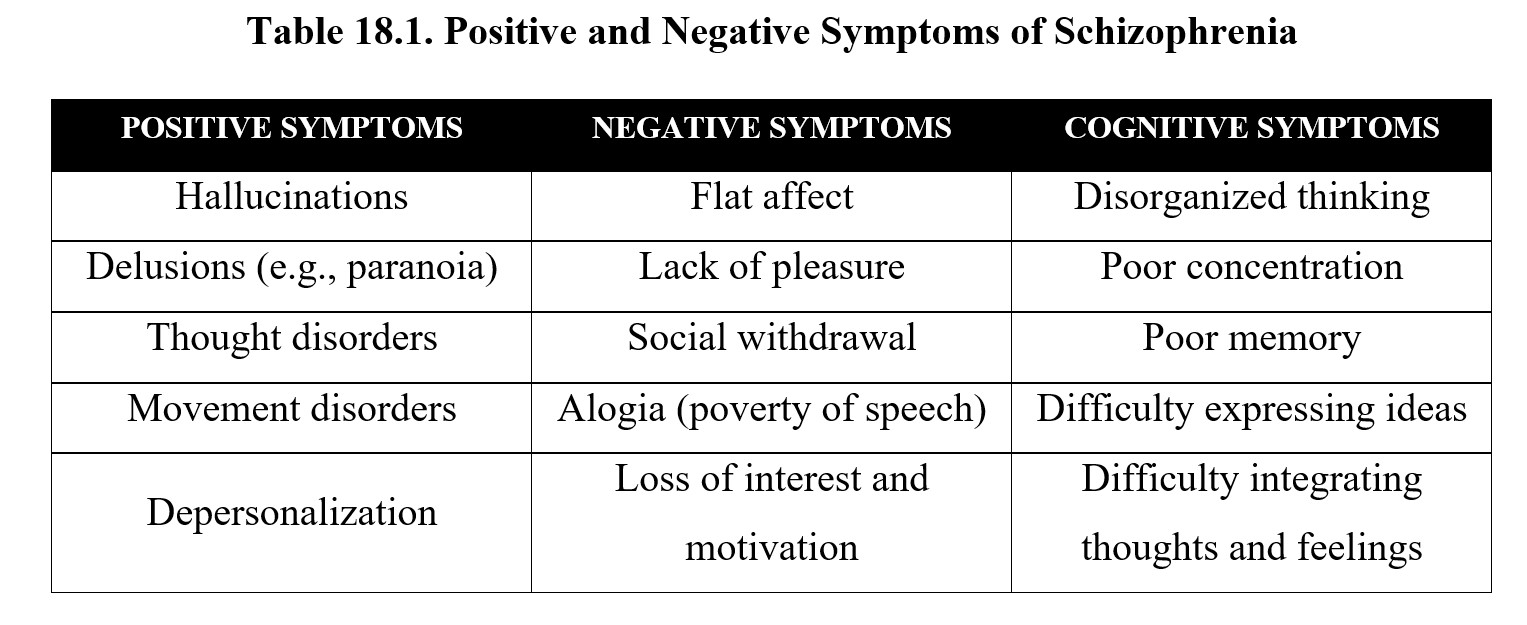
Many of the positive symptoms listed above may be familiar to you from the chapter on psychedelic drugs. This is because schizophrenia is a type of psychosis or altered perception of reality. Note that psychosis itself does not constitute schizophrenia. Schizophrenia involves other symptoms besides psychosis and is a chronic disorder that does not have to be precipitated by drug use.
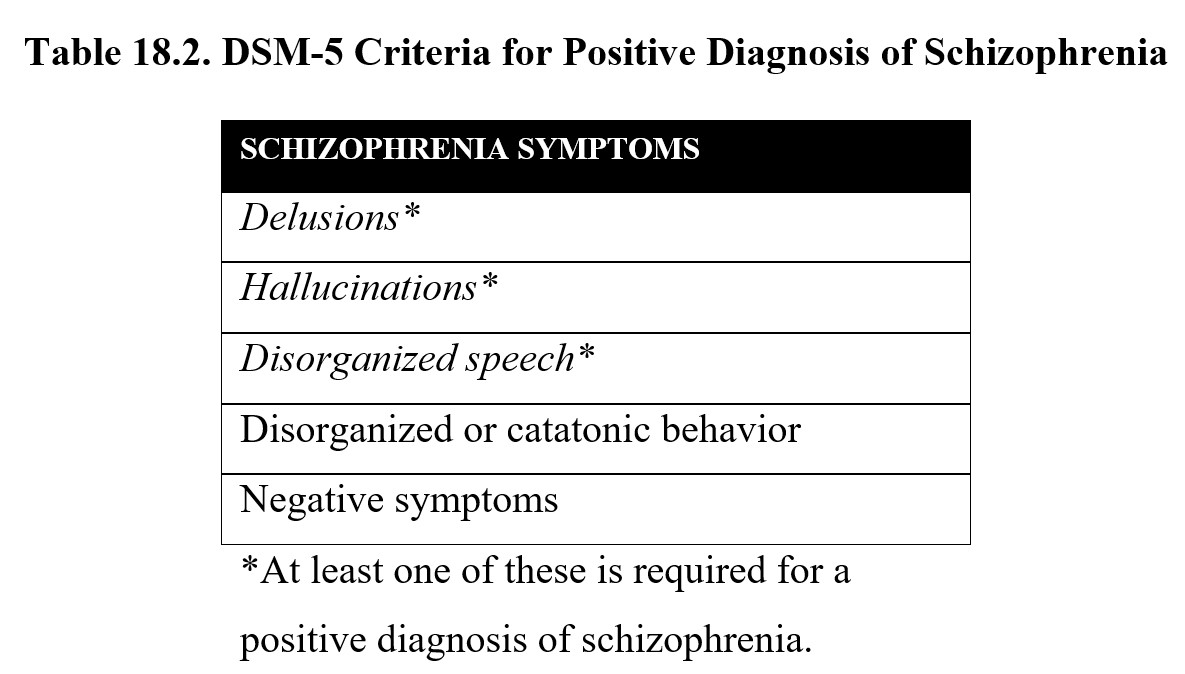
The DSM-V defines five main symptoms of schizophrenia (see below). Patients must have at least two of these symptoms, with one of the symptoms being either delusions, hallucinations, or disorganized speech. These symptoms must occur for at least six months, including one month of active symptoms.
Schizophrenia is less common than the other mental illnesses that we have covered so far in this unit, with a worldwide prevalence of 0.30–0.66%. It occurs more often in men than women. Men also tend to have an earlier onset and more severe symptoms compared to women.
The connection between schizophrenia and violence has often been sensationalized in the news and media. This results in a stigma towards schizophrenia and other mental illnesses. Some studies show that most people with schizophrenia are not violent and that instances of violence perpetrated by people with schizophrenia are rare (Silverstein et al., 2015). However, research has also shown that cases of people with schizophrenia committing violent acts have more to do with increased risk for substance abuse disorders in schizophrenic populations. Because the stigma can deter people from treatment and result in poorly informed public health policy, this association between schizophrenia and violence is highly damaging and harmful.
18.1.2. Causes of Schizophrenia
Because schizophrenia is a family of related disorders and can vary depending on the individual, it is difficult to pin down an absolute cause. Instead, it is better to think of schizophrenia as something that arises due to a variety of complex factors. Research has, however, provided some insight into why the symptoms of schizophrenia occur and how genetic vulnerability and environmental factors intersect.
To explore these ideas, we will start with the two-hit hypothesis, which states that schizophrenia requires two separate malfunctions in the brain. The first hit is a disruption in the early development of the CNS before birth. This first hit causes a long-term vulnerability to a second hit, which may occur in adolescence or adulthood and give rise to schizophrenia symptoms.
What might cause the initial disturbance in neurodevelopment? Research points to multiple factors. Faulty genes may play a role since research has shown that having relatives with schizophrenia increases the risk of developing the disorder. Prenatal conditions, such as low oxygen, maternal stress, or exposure to the influenza virus during pregnancy can also result in an increased risk.
Neuroimaging studies have indicated that people with schizophrenia have decreased volume of the left hemisphere, temporal lobe, prefrontal cortex, and thalamus (Ross et al., 2006). The exact mechanism of these changes is not entirely known, although it is suspected that they occur due to the disorganization of circuitry in the brain. In short, the connections between different areas become disorganized, leading to further structural changes during development.
The first hit alone is not sufficient for the development of schizophrenia. The second hit involves subjecting the brain to some sort of stress that it is unable to process correctly. This causes disrupted signaling in the CNS and leads to symptoms characteristic of schizophrenia, such as hallucinations and delusions. This may also explain why schizophrenia is a progressive disease that worsens over time if not treated. The adverse symptoms of schizophrenia may induce further disruptions to signaling and accelerate pathological changes.
18.1.3. Dopamine Hypothesis of Schizophrenia
One factor that we did not cover above is the role that neurotransmission plays in the expression of schizophrenia. There are two major hypotheses for how neurotransmission may be related. We will begin by looking at dopamine, the first transmitter found to be implicated in schizophrenia. Examine the diagram below:
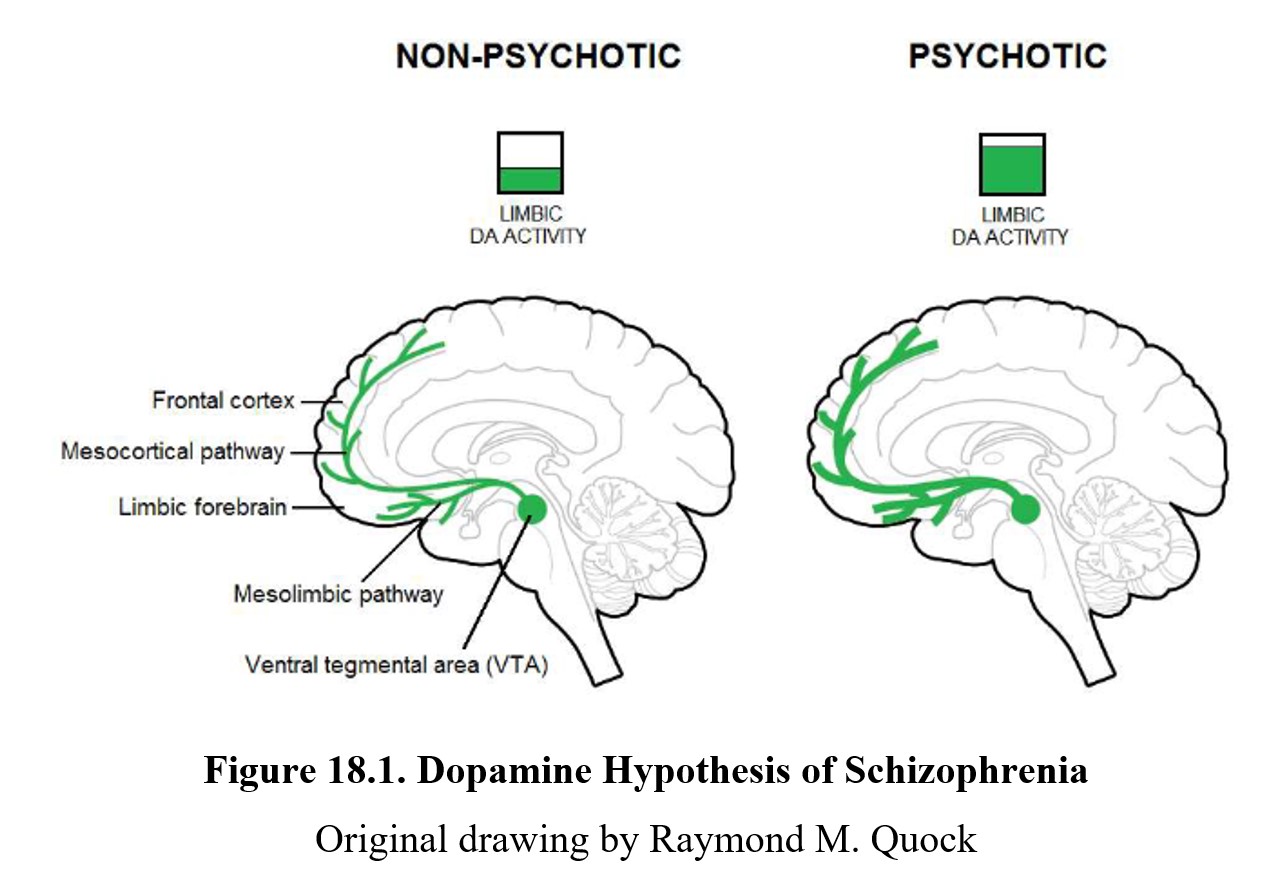
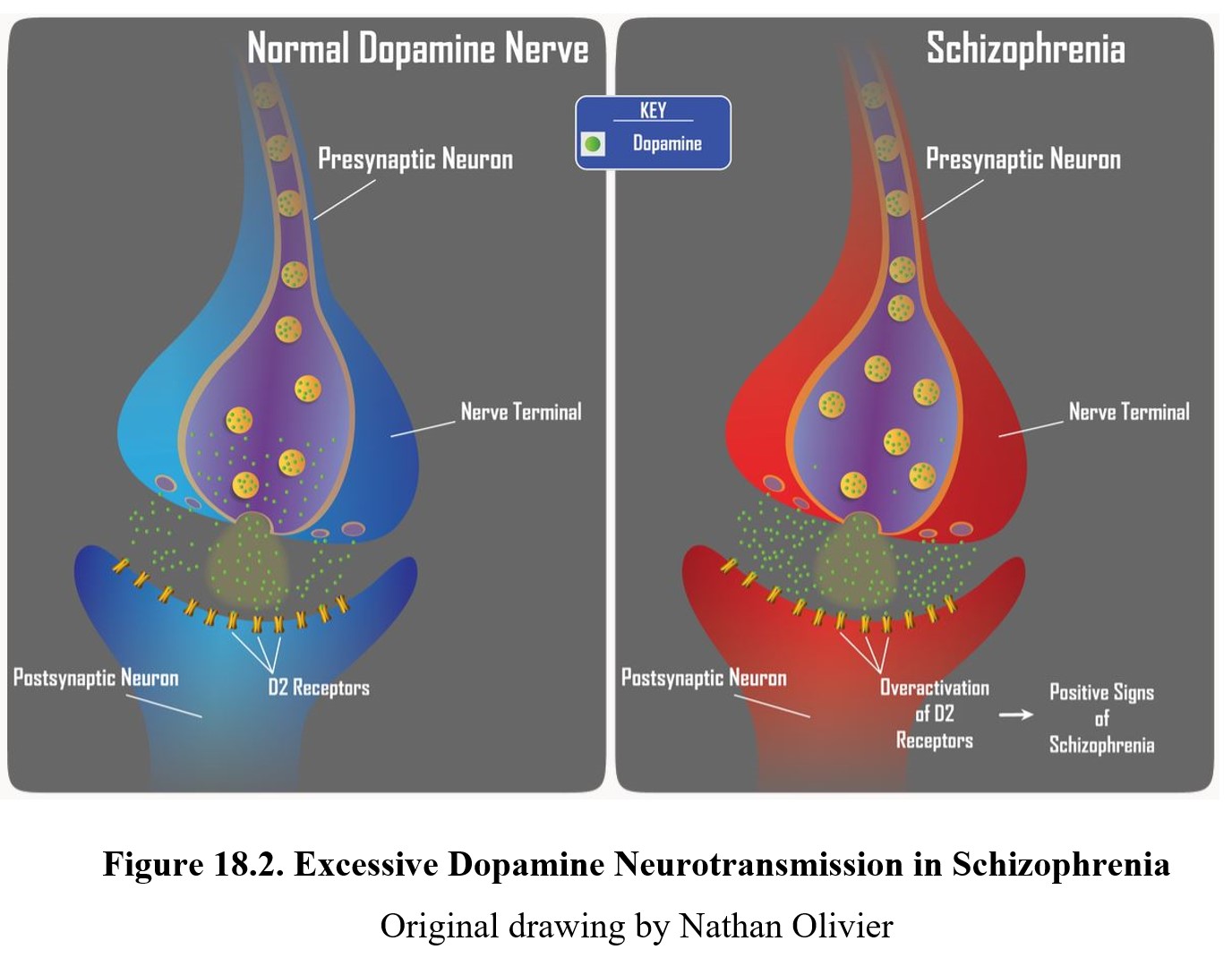
As you should recall from Chapter 7, dopamine is involved in pathways involved in the reward system such as the mesolimbic and mesocortical pathways, highlighted above. Schizophrenia is thought to be linked to high levels of dopamine activity in these areas because many of the first antipsychotics were antagonists of the D2 receptor. This implied that schizophrenia symptoms, especially positive signs, were caused by the overactivation of D2 receptors in the limbic system (see below).
18.1.4. Glutamate Hypothesis of Schizophrenia
The second neurotransmitter associated with schizophrenia is glutamate. You have already heard about this hypothesis during the chapter on psychedelics. Recall that drugs such as PCP and ketamine, which interfere with glutamate transmission, can cause episodes of psychosis very similar to what is seen in schizophrenia.
Although it is listed as a separate hypothesis, glutamate’s effect on psychosis is closely related to the dopamine story. Glutamate, an excitatory neurotransmitter, can innervate GABAergic neurons in the limbic system. These neurons in turn inhibit dopamine activity. In comparison to normal functioning (left panel below), people with psychosis (right panel below) exhibit low levels of glutamate and GABA activity. This disinhibits, or increases, dopamine activity.
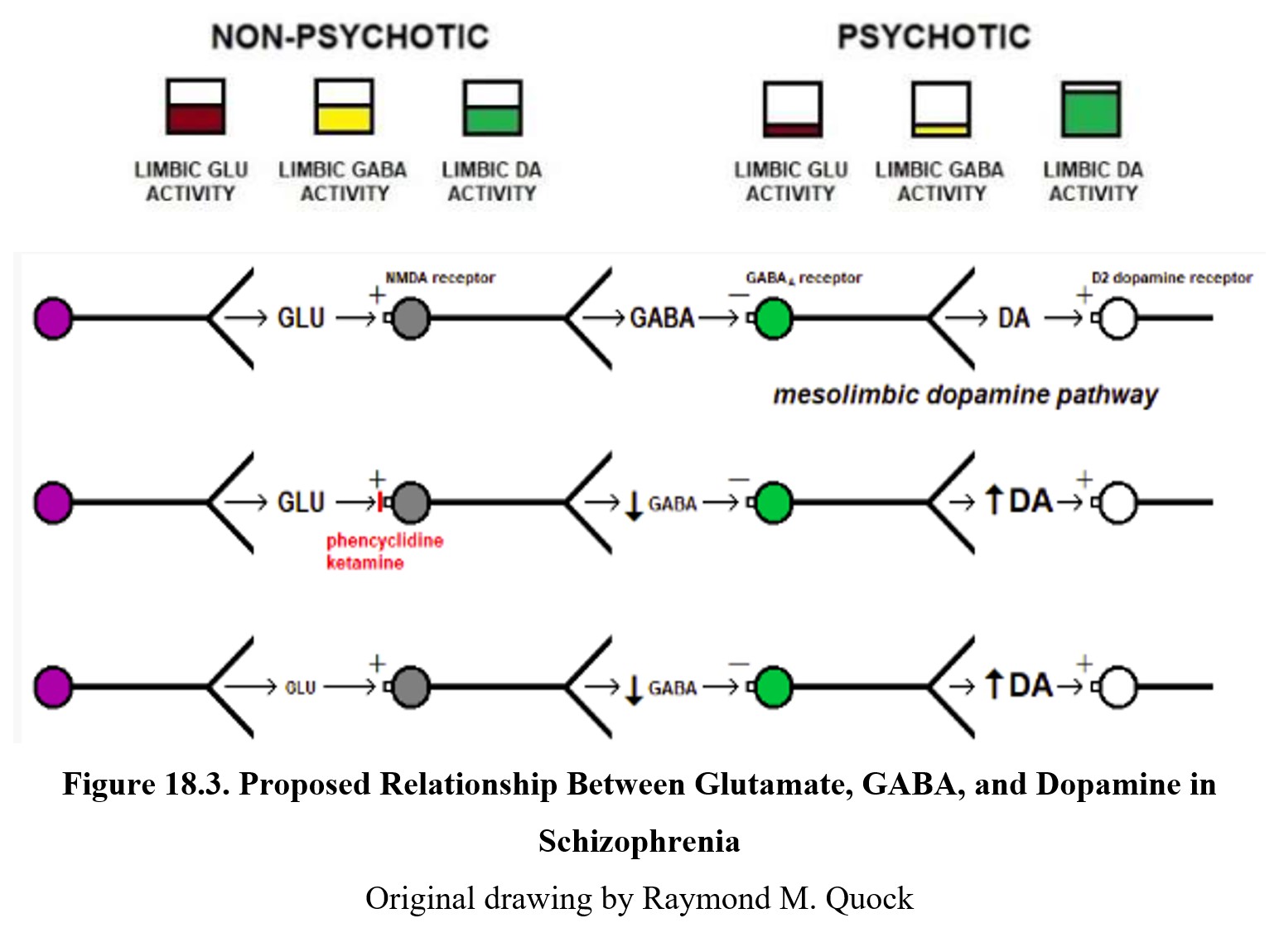
You can also see this relationship in the diagram above. The first row shows normal signaling. Glutamate released by the neuron on the left activates NMDA receptors on a GABA neuron, leading to GABA release. This binds to the GABAA receptor on a dopaminergic neuron (green), which inhibits dopamine release.
The second row shows what happens during PCP- or ketamine-induced psychosis. These drugs are antagonists at the NMDA receptor, so glutamate transmission is reduced and there is less glutamate inhibition of GABA release. The diminished GABA transmission results in the dopaminergic neuron being disinhibited and excessive dopamine release. The increased activation of dopamine receptors results in psychotic behavior. In the third row, you can see how low glutamate levels caused by schizophrenia produce the same results.
18.2. Antipsychotic Drugs
Section Learning Objectives
- Describe the history of schizophrenia treatment and antipsychotic drugs.
- Contrast different routes of administration for antipsychotics.
- Explain the mechanism of action of classical and atypical antipsychotics.
- Explain the basis for extrapyramidal syndrome, tardive dyskinesia, and neuroleptic malignant syndrome.
- Discuss the use of chlorpromazine in drug abuse.
- Discuss the role of psychotherapy in the treatment of schizophrenia.
Antipsychotics, also known as neuroleptics, are drugs that reduce the symptoms associated with psychosis. Although they are primarily used in the treatment of schizophrenia, they may also be used to treat other disorders, such as being combined with mood stabilizers to treat bipolar disorder.
18.2.1. History and Overview
Early attempts at treating schizophrenia would be considered unethical by today’s standards. Common treatments included sustained restraint, isolation, ice baths, induced fevers, and shock therapy (Shorter, 1997). Perhaps the most infamous methods were lobotomies or surgeries where parts of the brain were removed, often in the frontal lobe. Although they did provide long-lasting treatment of schizophrenia, frontal lobotomies were ineffective at improving symptoms and not all patients survived the procedure.
The first antipsychotic drugs were developed in the 1950s. The discovery of chlorpromazine, the first neuroleptic drug, was an accident. It was originally developed as a mild anesthetic and administered to psychotic patients for sedation. Antipsychotic effects were observed and confirmed by multiple studies and Smith Kline & French began to market it as a psychiatric drug under the brand name Thorazine®.
Antipsychotic drugs can be divided into two broad categories, similar to antidepressants. The first group is the typical antipsychotics, also referred to as classical or first-generation antipsychotics. As the name suggests, these were the first antipsychotic drugs discovered. These drugs tend to be more effective at treating positive symptoms than negative ones. This group includes chlorpromazine, fluphenazine (Prolixin®), thioridazine (Mellaril®), and haloperidol (Haldol®).
Another class of neuroleptic drugs is referred to as novel or atypical antipsychotics, also referred to as second-generation antipsychotics. These drugs are distinguished by their slightly different pharmacological profiles, which allow for better treatment of negative symptoms. Drugs in this group include clozapine (Clozaril®), aripiprazole (Abilify®), risperidone (Risperdal®), and quetiapine (Seroquel®).
Compared to antidepressants, the differences between the two generations of antipsychotics are much smaller. Atypical antipsychotics reduce some side effects while introducing new ones. Because of this, typical antipsychotics are still a viable first-line treatment option for many people. A study showed that quality of life was similar between both groups of antipsychotics. We will revisit this comparison at the end of this section.
18.2.2. Administration and Pharmacokinetics
Antipsychotic medications are usually available in pill form and can be taken orally. This is not always ideal though, since some patients can have poor compliance when taking antipsychotics. As such, they are often injected intramuscularly, sometimes in the form of a depot injection. Depot injections allow for the slow release of the drug over several weeks from a single injection. Fluphenazine and haloperidol are examples of antipsychotics that can be administered through monthly depot injections.
The pharmacokinetics of antipsychotics depend heavily on the specific drug. Accordingly, there are large variations in bioavailability and half-life. Most antipsychotic medications are metabolized in the liver by cytochrome P450 enzymes and excreted mainly through the kidneys.
Chlorpromazine, the first antipsychotic discovered, has a wide range of possible bioavailability depending on the individual. It is primarily metabolized by CYP2D6 and has a half-life of around 30 hours. Because of its sedative effects, pairing chlorpromazine with other CNS depressants can cause a synergistic reaction and greater CNS depression than expected.
18.2.3. Mechanism of Action and Effects
The main effect shared by all antipsychotic drugs is a reduction in positive symptoms associated with schizophrenia. Recall that these are the symptoms associated with psychosis—hallucinations, delusions, and thought disorders. This antipsychotic effect is achieved by blocking D2 receptors and reducing dopamine transmission in the limbic system, consistent with the dopamine hypothesis of schizophrenia (see figure below).
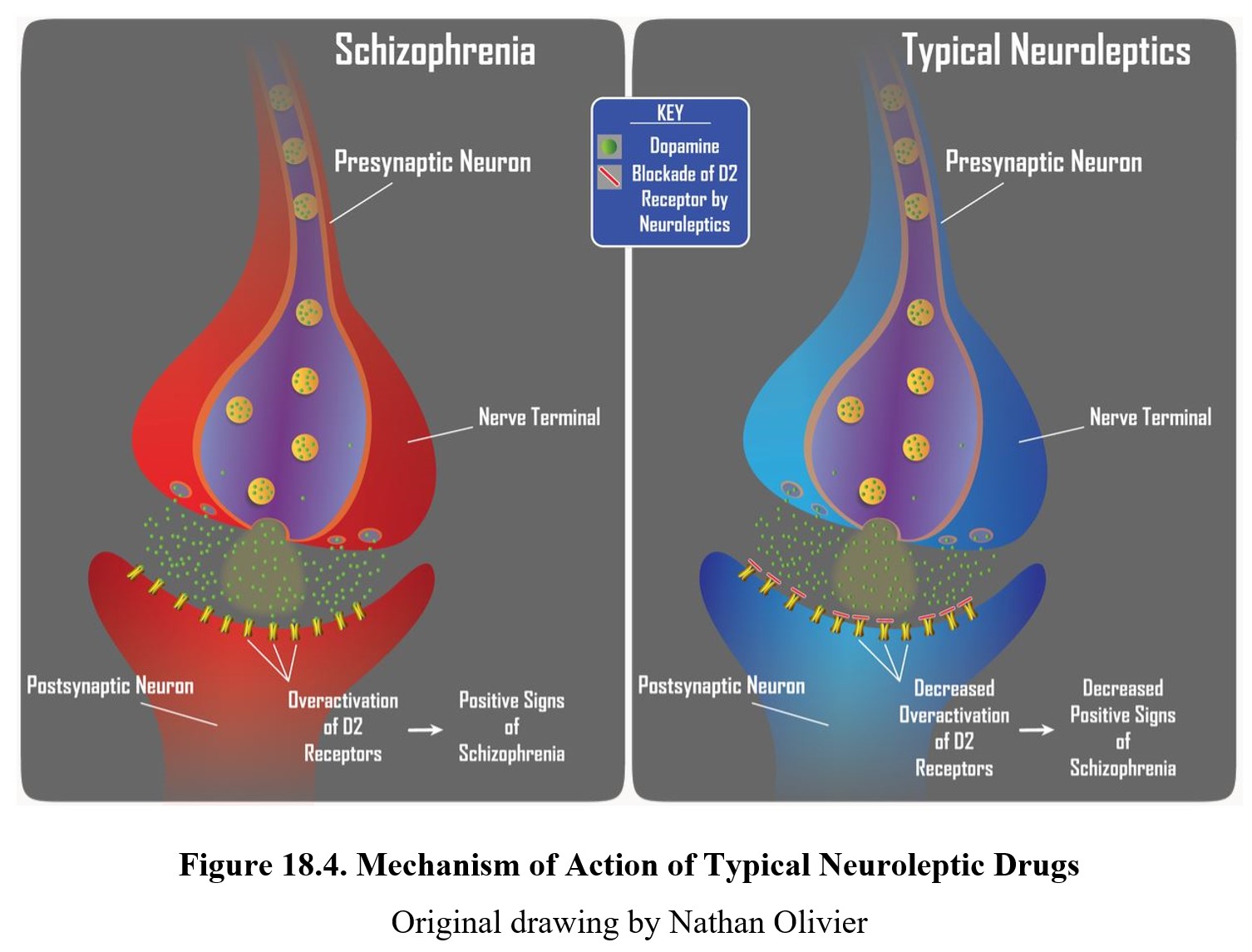
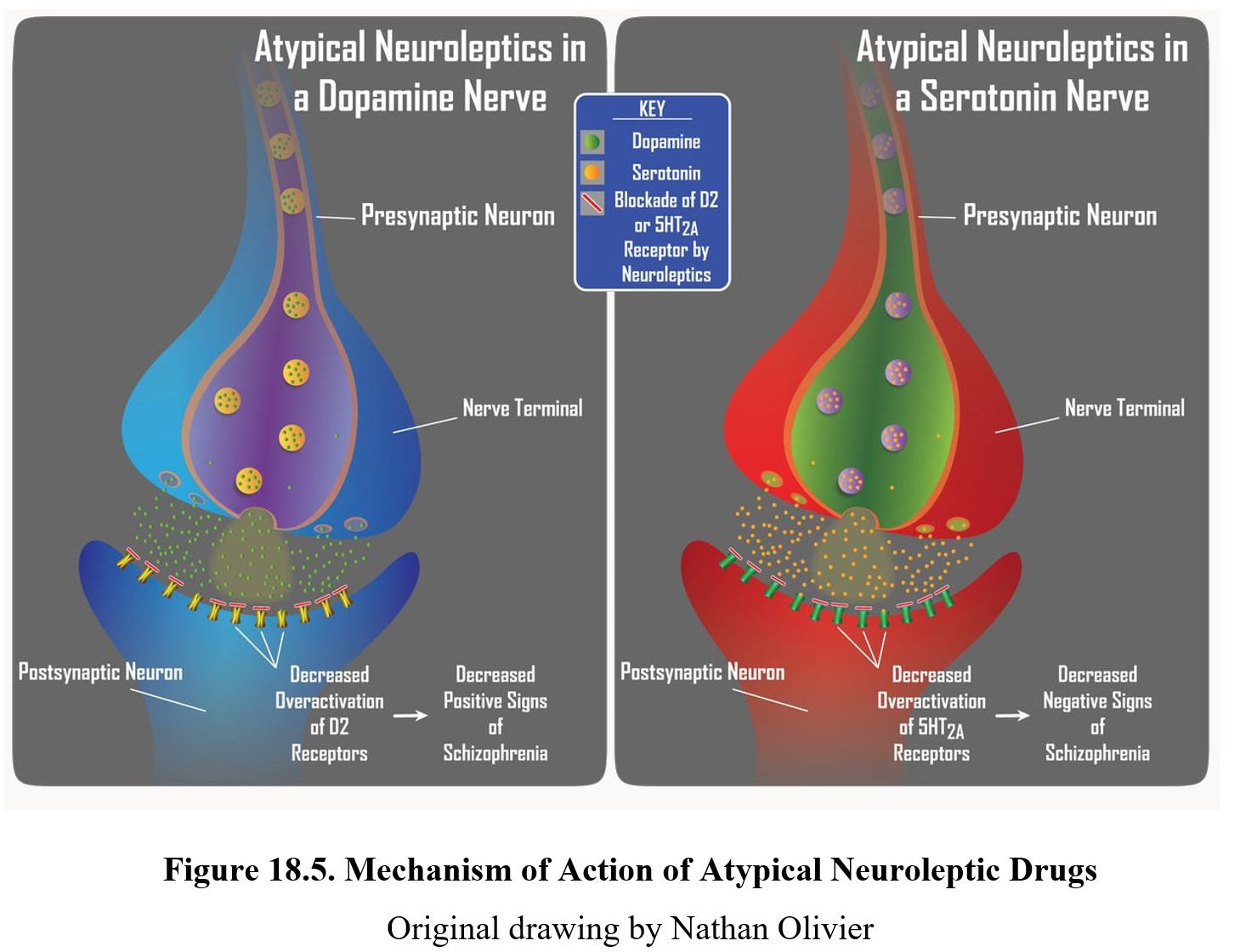
Atypical antipsychotics, such as clozapine, can reduce positive symptoms of schizophrenia and also have a greater effect on negative symptoms (e.g., flat affect, alogia, and lack of interest or pleasure). This is due to the dual mechanism of action of atypical neuroleptics. Similar to typical neuroleptics, these second-generation drugs retain the ability to block D2 receptors to reduce positive signs. Initially, they were considered atypical because they produced minimal extrapyramidal motor disturbance. Subsequently, it was noted that these neuroleptics were more effective than first-generation drugs in reducing negative signs of schizophrenia, which are largely mediated by serotonin receptors in the limbic system.
The figure below shows the neurochemical effects of atypical antipsychotics on dopamine (panels on the left) and serotonin (panels on the right) transmission. Positive symptoms of schizophrenia are associated with the overactivation of D2 receptors (upper left panel), while negative symptoms are associated with the overactivation of 5-HT2A receptors (upper right panel). Atypical neuroleptics are antagonists at both of these receptors, which provides them with a broader spectrum of antipsychotic activity than typical neuroleptic drugs.
Antipsychotic drugs share similar side effects. As was the case with antidepressants and anxiolytics, antipsychotics may produce sedation, anticholinergic effects, and orthostatic hypotension. Antipsychotics are also antiemetics, or drugs that reduce vomiting. Typical antipsychotics do not usually increase weight gain, but atypical ones have a greater risk for weight gain or diabetes. There are other more significant side effects of antipsychotics, but we will discuss those separately in the next subsection.
Tolerance does not develop for antipsychotic effects, although it may develop for some side effects such as sedation. Although dependence and addiction do not develop in the traditional sense, upon cessation of antipsychotic drugs, patients may experience discontinuation symptoms. These symptoms include nausea, vomiting, diarrhea, increased heart rate, dry mouth, anxiety, and insomnia.
18.2.4. Other Adverse Effects
As mentioned previously, antipsychotics come with some adverse side effects. The first we will discuss is an extrapyramidal syndrome (EPS), a group of movement disorders associated with dopamine antagonists. The effects resemble Parkinson’s disease and include tremors, rigidity, and slow movement. Other disorders include dystonia, or continuous muscle contractions, and akathisia, or a sense of restlessness.
The cause of EPS is reduced dopamine activity in the nigrostriatal pathway (see figure below). In schizophrenia, there is a high level of dopamine transmission in the mesolimbic and mesocortical pathways (left lower panel below). This excessive activity can be counteracted by blocking D2 receptors with an antipsychotic drug. However, this also reduces dopamine transmission in the nigrostriatal pathway (right lower panel below), which can give rise to extrapyramidal side effects.
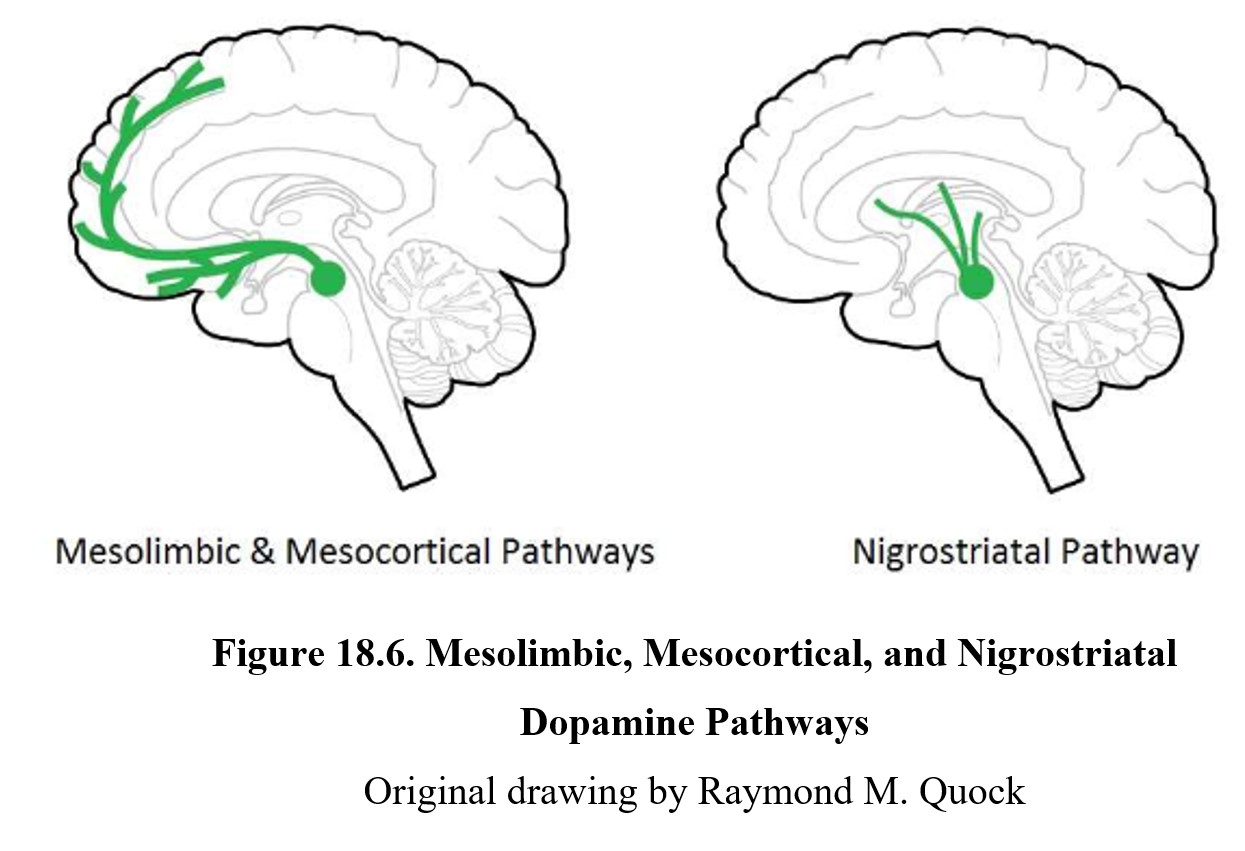
The reason why EPS and Parkinson’s disease are so similar is that both involve the under-activation of dopamine receptors in the striatum. Compare the two in the image below. In Parkinson’s disease, reduced dopamine transmission is the result of chronic degeneration of dopaminergic neurons. The underactivation of striatal dopamine receptors results in the tremors, rigidity, and bradykinesia that are so characteristic of Parkinson’s disease. In EPS, it is the blockade of D2 receptors by neuroleptic drugs that reduces dopamine transmission in the nigrostriatal pathway. The underactivation of dopamine receptors due to the receptor blockade again results in tremors, rigidity, and bradykinesia. Though the causes are different, the reduction in dopamine neurotransmission is the same and leads to similar neurological signs. This is why neuroleptic drugs are said to produce a Parkinson-like neurological side effect.
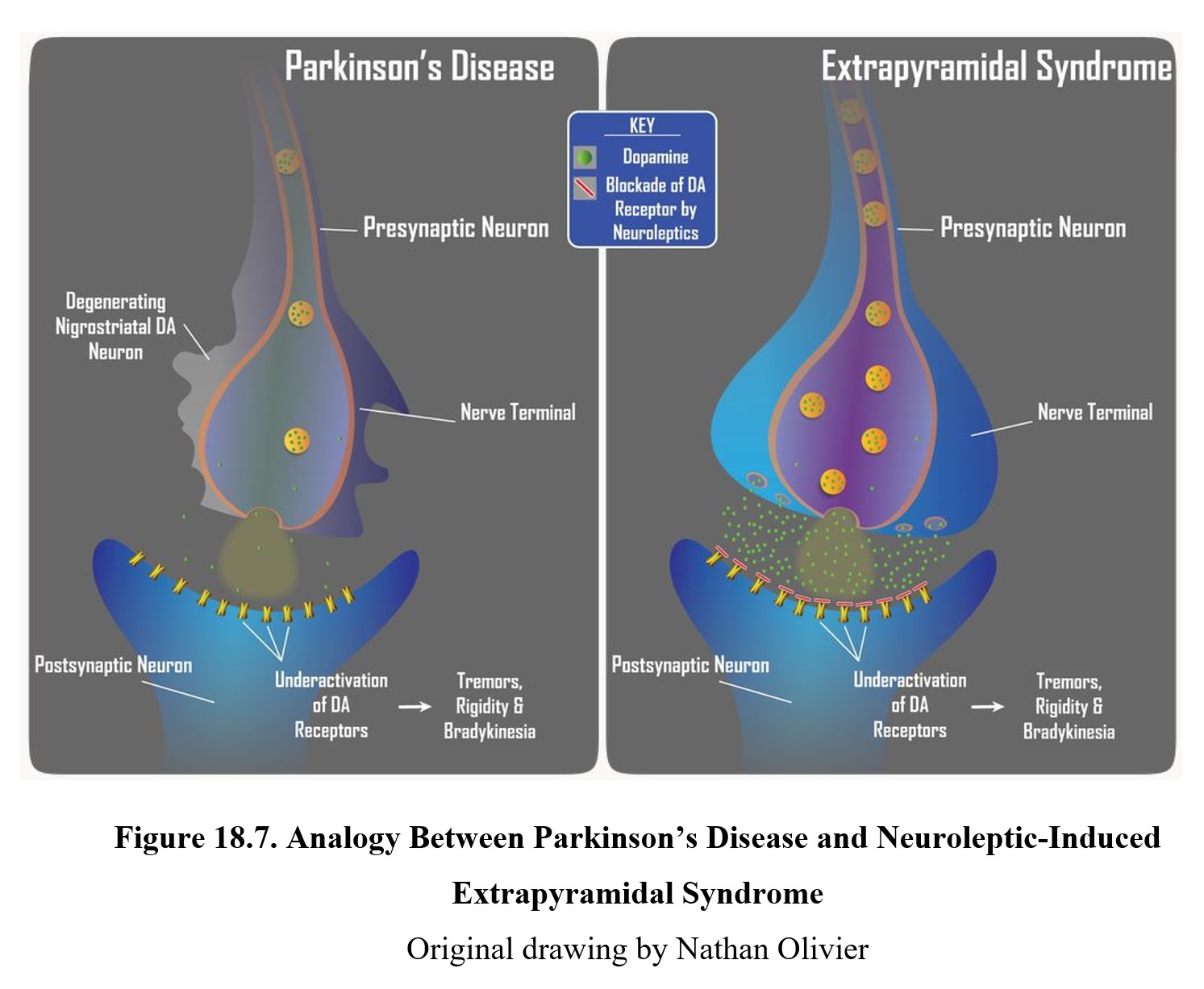
Extrapyramidal side effects are typically reduced in atypical antipsychotics. The lack of EPS was one of the main reasons for distinguishing atypical antipsychotics from typical ones. EPS is also one of the major reasons why patients stop taking antipsychotics or drop out of clinical trials.
Another severe side effect is tardive dyskinesia (TD), a disorder that involves involuntary and repetitive movements of the cheeks, face, tongue, and jaws. Its characteristics are the opposite of EPS. Common actions involve grimacing, sticking out the tongue, and smacking or pursing the lips. It is a severe disorder that can be embarrassing and interfere with daily life.
Presentation of Various TD Symptoms [2:10]
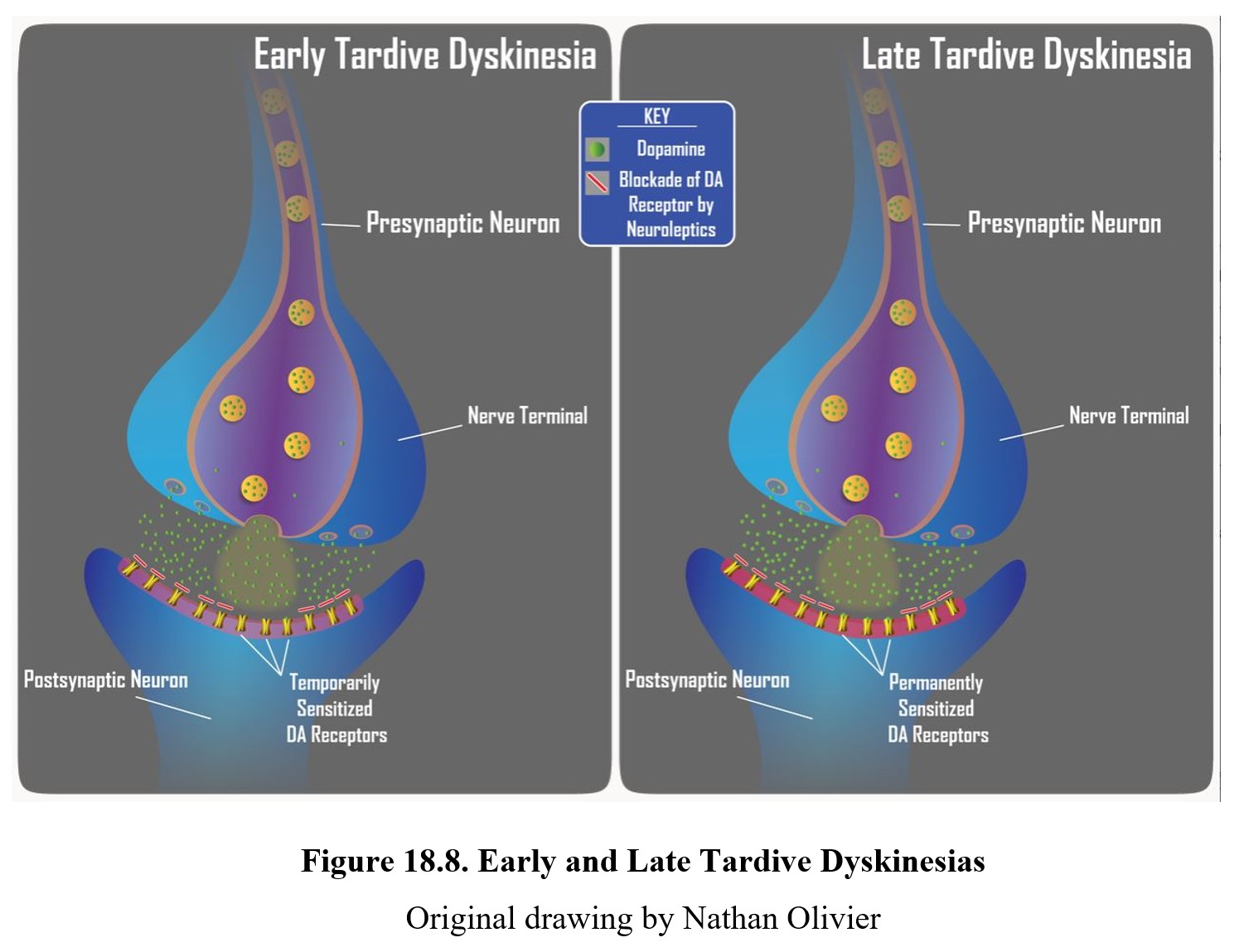
TD can develop in some patients during long-term, high-dose therapy. It is more common with typical antipsychotics, although treatment with some atypical antipsychotics can still result in TD. The cause appears to be the chronic blockade of dopamine receptors, which results in upregulation or sensitization of the receptors (see below). Thus, tardive dyskinesia is characterized by dopamine hyperactivity. Signs of TD typically appear when the dose of neuroleptic is reduced or medication is changed. If this is caught early (upper panel below), the signs of TD can be reduced by increasing the dose of the neuroleptic drug. However, if TD becomes firmly established (lower panel below), it may become permanent and persist for months or years after discontinuation of antipsychotics.
Tardive Dyskinesia Symptoms and Information [2:42]
One final adverse side effect to mention is a neuroleptic malignant syndrome (NMS). This is a rare but potentially life-threatening condition that resembles the flu. Symptoms include sweating, fever, muscle rigidity, and changes in blood pressure, heart rate, and breathing rate. It typically occurs in the first few weeks of treatment. The cause seems to be related to dopamine receptor blockade, similar to TD, although there may be other factors involved as well. Similar to TD, NMS occurs slightly more often in typical antipsychotics, although both types can cause NMS (Berman, 2011).
Why do typical antipsychotics show higher rates of EPS and slightly higher rates of TD and NMS? It is believed that one of the other ways that atypical antipsychotics differ from their predecessors is a fast dissociation from dopamine receptors. In other words, drugs like clozapine bind to D2 receptors for only a short time and rapidly dissociate, a phenomenon referred to as the “kiss and run” hypothesis. This brief binding time may reduce the rate and severity of the neurological side effects caused by the antagonism of extrapyramidal dopamine receptors.
18.2.5. Comparison of Antipsychotic Drugs
Before we conclude this chapter, let us briefly discuss the roles of different antipsychotics. Chlorpromazine has long been considered a universal antidote for drug-induced psychosis. This is because it can competitively block multiple different receptors, including those for dopamine, norepinephrine, acetylcholine, histamine, and serotonin. This classifies chlorpromazine as a “dirty drug” similar to ethanol because of its widespread effects.
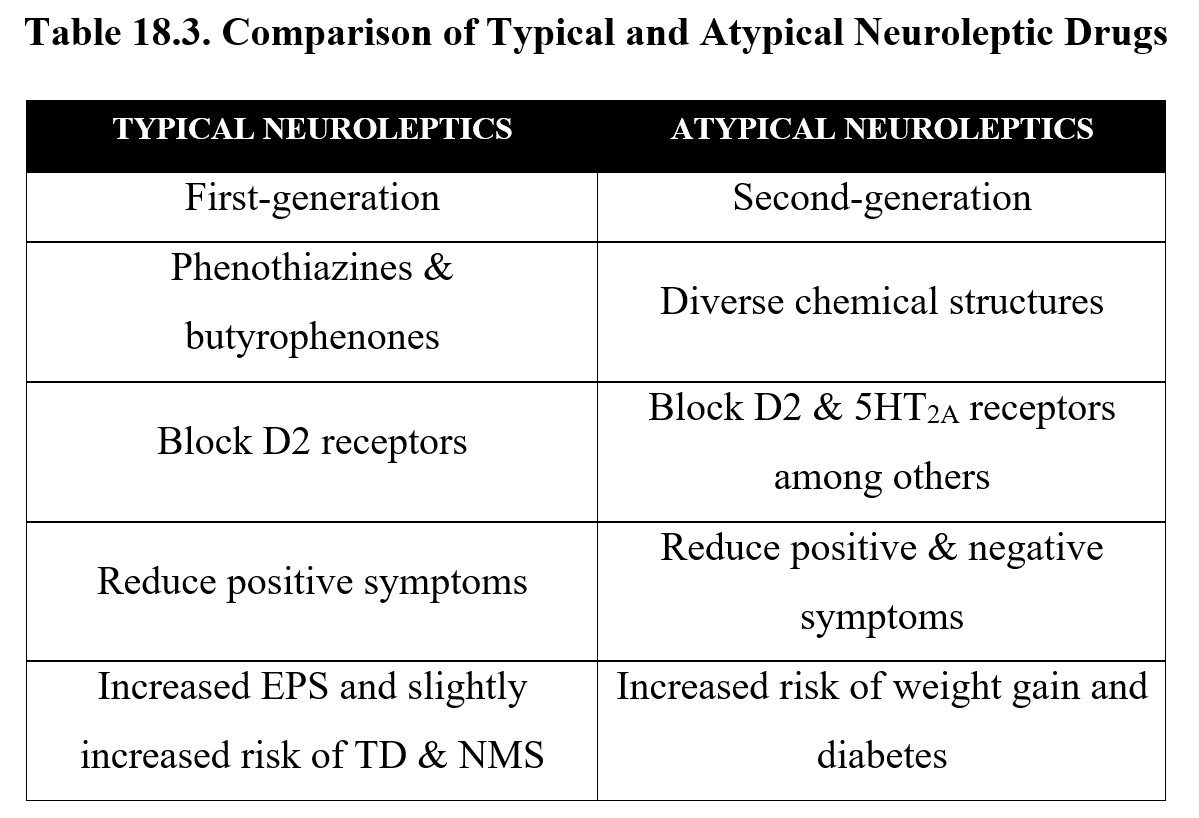
Although we have distinguished between typical and atypical antipsychotics throughout this chapter, it is important to remember that atypical antipsychotics present at most, moderate improvement over typical antipsychotics. Typical neuroleptic drugs represented by two chemical classes (phenothiazines and butyrophenones) may still be preferred in some cases when balancing individual symptoms, compliance, and potential side effects. Atypical neuroleptics may be preferred if there are prominent negative signs of schizophrenia. The principal differences between the two groups are listed below, along with examples of each:
Finally, it is worth mentioning that the most effective treatment plans combine drug therapy with psychotherapy, which can teach patients social skills and decrease social isolation. Pharmacotherapy accompanied by psychotherapy is more effective than pharmacotherapy alone. The converse is also true, psychotherapy with pharmacotherapy is more effective than psychotherapy alone. Psychotherapy may also be useful for dealing with the side effects of antipsychotic medications, such as increased weight gain.
Chapter Review and Summary
In this chapter, we learned about schizophrenia and antipsychotics. We began with the symptoms and diagnostic criteria of schizophrenia before moving on to its potential causes and neurochemical hypotheses. We then examined the drugs used to treat psychosis, which can be grouped into typical and atypical antipsychotics. We compared the actions and effects of both types, including adverse side effects such as extrapyramidal syndrome, tardive dyskinesia, and neuroleptic malignant syndrome. Finally, we discussed the role of chlorpromazine as an antidote for drug-induced psychosis and how psychotherapy can contribute to the treatment of schizophrenia.
Good work on making it this far. Stay focused and be sure to ask your instructor or teaching assistant for help if you need it.
Chapter 18 Practice Questions
Answer the following questions:
- What is the difference between positive and negative symptoms of schizophrenia?
- What are the five symptoms mentioned by the DSM-V for diagnosing schizophrenia?
- Describe the two-hit hypothesis. Provide three examples of events or factors that could contribute to the first hit.
- Compare the dopamine and glutamate hypotheses of schizophrenia. Are the two hypotheses contradictory?
- What was the first antipsychotic drug discovered?
- How do the mechanisms of action of typical and atypical antipsychotics differ? What effects are these associated with?
- Why are antipsychotics sometimes injected instead of taken in pill form?
- What disease does extrapyramidal syndrome resemble?
- What causes tardive dyskinesia?
- Are atypical antipsychotics clear improvements over typical antipsychotics? Explain.
2nd edition