Chapter 12: Opioids
2nd edition as of August 2022
Chapter Overview
In the previous unit, we covered stimulants and depressants. In this third unit, we will explore three more classes of drugs: opioids; cannabinoids; and psychedelics. The first of those, opioids, is the subject of this chapter. Many different opioids have been discovered or created, but all act on opioid receptors and have similar effects. In the following sections, we will take a look at some of the most common opioids and compare their properties and uses.
Chapter Outline
- 12.1.1. Types of Opioids
- 12.1.2. History and Legislation
- 12.1.3. Opioid Receptors
- 12.2.1. Absorption and Distribution
- 12.2.2. Metabolism and Excretion
- 12.3.1. Pain Relief
- 12.3.2. Other Effects
- 12.3.3. Tolerance and Overdose
- 12.3.4. Dependence, Withdrawal, and Treatment
Chapter Learning Outcomes
- Describe types of opioids and the history of legislation regarding opioids.
- Describe the pharmacokinetics of opioids.
- Describe the pharmacodynamics of opioids.
- Describe opioid use disorder.
- Outline treatment options for opioid dependence.
12.1. Opioids Overview
Section Learning Objectives
- Differentiate between the three classes of opioids and provide examples of each.
- Explain the history of opioid use and the current opioid epidemic.
- Describe the endogenous opioid system and the three major endogenous opioid peptides and opioid receptor subtypes.
The term opiate originally referred to alkaloids extracted from the opioid poppy, including morphine and codeine. With the emergence of semisynthetic and synthetic compounds that mimicked the effects of morphine, they became known as opioids because they resembled opiates. But today the terms opiate and opioid are used interchangeably as opioid has become a blanket term for all drugs with pharmacological properties similar to morphine.
Another common description of these drugs is narcotic (narkoun in Greek for “to make numb.”). The origin of the word was based on the ability of opioid drugs to cause sedation and sleep. However, the Federal government continues to use the term narcotic synonymous with illicit. For that reason, cocaine, which is not a narcotic, remains listed in Federal law as a narcotic.
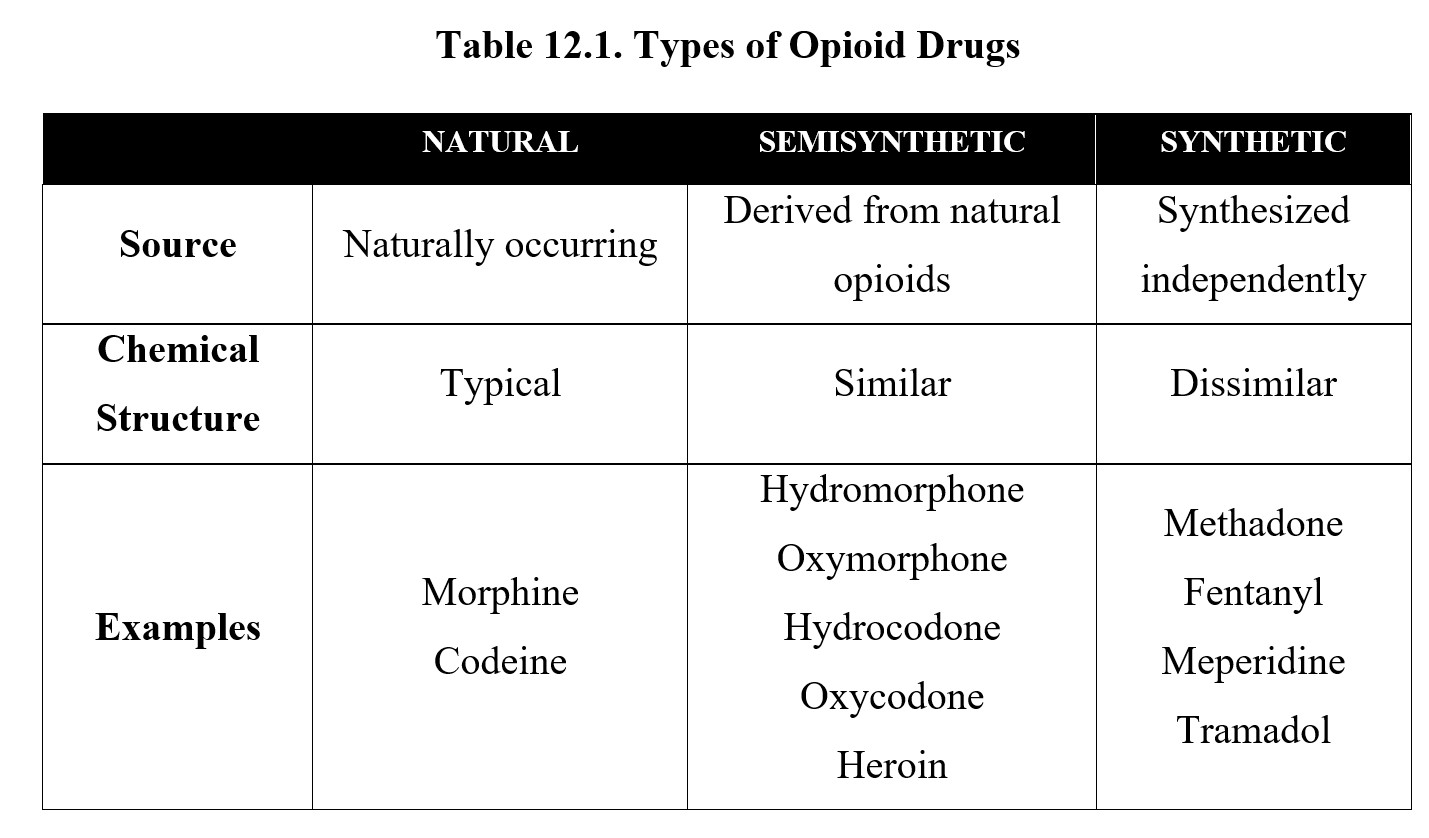
12.1.1. Types of Opioids
Opioids can be classified into three different types. Natural opioids are opium alkaloids that have a phenanthrene nucleus found in the opium poppy plant (Papaver somniferum). They are also referred to as opiates. The three most important opiates are thebaine, morphine, and codeine. Thebaine is not used clinically but serves as a precursor to many semisynthetic opioids. Morphine and codeine are useful as clinical analgesics. Of the two, morphine is stronger and has greater effects. Because of this, morphine is the prototypical opioid and the benchmark against which other opioids are measured. Codeine is considered a weak analgesic drug and is used more for its antitussive property.
The drugs that are synthesized from natural opioids are called semisynthetic opioids and have similar chemical structures to opiates. Perhaps the most notable semisynthetic opioid is heroin, also called diacetylmorphine or diamorphine, the generic name in the United Kingdom. The names of most semisynthetic opioids will reflect which substance they are most chemically related to. The names hydromorphone (Dilaudid®) and oxymorphone (Opana®) are reminiscent of morphine, while hydrocodone (Vicodin®) and oxycodone (Percocet®, OxyContin®) are more similar to codeine.
Finally, synthetic opioids are synthesized independently within a lab instead of being based on naturally occurring opiates. These do not share the phenanthrene nucleus of opiates or semisynthetic opioids but can, nonetheless, couple to the opioid receptor and produce similar pharmacological effects. Synthetic opioids include methadone, fentanyl, meperidine, and tramadol. See the table below for a summary of the different types.
12.1.2. History and Legislation
Opium has been used for thousands of years in medicines and religious rituals. The earliest evidence of human use dates back to 5000 B.C. in the Mediterranean area, although by ancient times, it had spread throughout Asia, Europe, and Africa. The first documented recreational use of opium occurred in China during the 15th century. Shortly after, there were reports of opium addiction. The production and trade of opium flourished. In the 1800s, in response to attempts by the Chinese government to reduce opium use and smuggling, Britain and France fought wars to ensure the continued trade and consumption of opium.
The main active component of opium, morphine, was isolated in the early 1800s by German pharmacist Friedrich Sertürner, who named it after Morpheus, the Greek god of dreams. It was marketed as a pain medication and treatment for opium addiction, although it was soon discovered to have addictive properties itself. Morphine was also used regularly in surgical procedures following the invention of the hypodermic needle in the 1850s.
Heroin was developed in 1898 and marketed by Bayer the German pharmaceutical company as a cough suppressant and non-addictive alternative to morphine. It was not long until this “non-addictive” claim was found to be untrue. In response to rising rates of opium, morphine, and heroin use, the Harrison Narcotics Act of 1914 effectively banned their prescription and use. As a result, opioid use was stigmatized, and physicians generally avoided prescribing opioids to patients.
In recent decades, however, the U.S. has seen an opioid epidemic. The epidemic began in the 1990s when certain pharmaceutical companies made a major push to reduce the stigma around opioids and increase prescription rates. Through aggressive marketing and misinformation during this period, the safety of chronic opioid use was exaggerated, and many semisynthetic and synthetic opioids were developed and widely prescribed as painkillers, such as oxycodone (OxyContin®). If you are interested in learning more about this, check out this article:
Business Insider | This one-paragraph letter may have launched the opioid epidemic
This letter to the editor was continuously cited by drug companies, scientific societies, and physicians as justification for the more liberal use of opioids for the treatment of any type of pain without fear of addiction. However, the database included patients administered low doses of opioids in a hospital setting to ease acute pain rather than out-patients given long-term opioid treatment to relieve chronic pain. This overgeneralization was deceptive yet convincing to the public and the medical community that opioid drugs were minimally addictive.
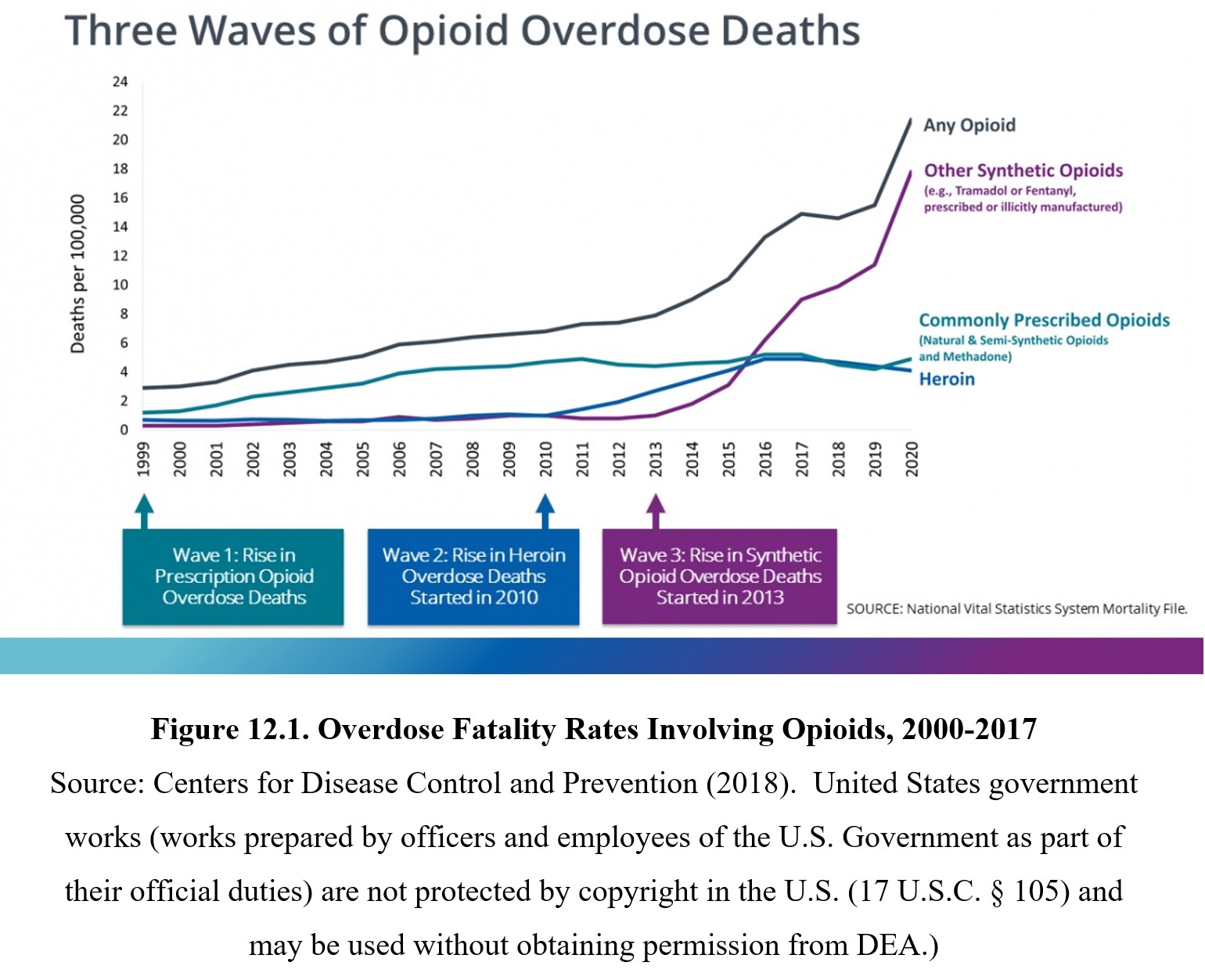
Due to the increase in opioid prescriptions, overdose deaths related to opioid use hit all-time highs, totaling 400,000 deaths in the U.S. from 1999 to 2017. Part of this is because people started by misusing opioid painkillers but moved on to more potent drugs when supply was restricted. Examine the graph below. You will notice that deaths related to prescription opioid use increased first, then heroin, then synthetic opioids like fentanyl.
Various opioids are placed under different schedules because some have greater potential for dependency and addiction. Most opioids are Schedule II because they have high abuse potential but also have accepted medical uses. These include morphine, codeine, oxycodone, fentanyl, and many other opioid pain relievers. Heroin is classified as a Schedule I drug with no accepted medical use. Some medicines containing codeine are classified in lower schedules, such as codeine-containing Tylenol® (Schedule III) or cough medicines (Schedule V).
12.1.3. Opioid Receptors
Opioids are defined by their effects on opioid receptors. There are three main opioid receptor subtypes: the mu-opioid receptor, delta-opioid receptor, and kappa-opioid receptor. By taking the first letter of each Greek letter (μ, δ, and κ), it is also common to abbreviate them as either MOP, DOP, and KOP (with OP = opioid), or as MOR, DOR, and KOR (with OR = opioid receptor). Different terminologies are used by different teachers and scientists. Researchers have discovered other receptors in the opioid receptor family, but for this class, we will focus on these three subtypes.
All opioid receptors on neuronal tissues are inhibitory 7TM G-protein coupled receptors (GPCRs). Opioid receptors can be presynaptic, where they inhibit the release of neurotransmitters, or postsynaptic, where they hyperpolarize the postsynaptic neuron.
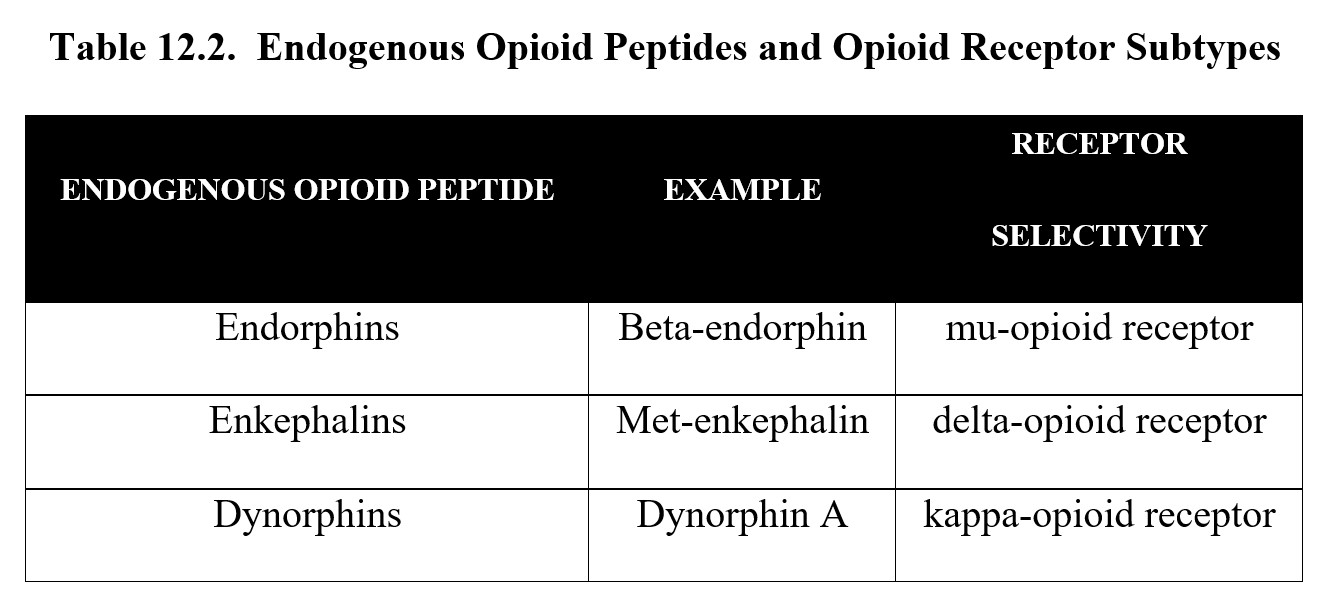
In the chapter on neurotransmitters, we discussed endorphin or endogenous morphine. Endorphins are classified as endogenous opioid peptides, which are naturally-occurring chains of amino acids that activate can opioid receptors. (Recall that a peptide is simply a chain of amino acids, but they have the right configuration to fit the receptor). Endorphins are one of the three major types of opioid peptides, the other two being enkephalins and dynorphins as well as additional peptides. Although their role is in pain and pain relief, endogenous opioid peptides are involved in many other physiological functions as well.
Each opioid peptide has a different affinity and efficacy for opioid receptor subtypes. Thankfully, the overall pattern is fairly straightforward. Endorphins tend to target mu-opioid receptors, enkephalins tend to target delta-opioid ones, and dynorphins tend to target kappa-opioid receptors. Although they are relatively selective for certain receptor subtypes, this does not mean they cannot activate other subtypes or even other non-opioid receptors. See the following table for comparison:
So, what is the difference between the opioid receptor subtypes? The effects commonly associated with opioids such as pain relief and euphoria are mediated primarily through the mu-receptor and, to a lesser extent, the delta-receptor. The kappa-receptor is interesting in that it contributes to increased pain sensitivity through dysphoria while also evoking spinal analgesia.
Recall that in Chapter 4, we also introduced Substance P, a neurotransmitter responsible for transmitting pain signals to the CNS. Because opioid receptors are inhibitory, their activation tends to suppress the release of Substance P and other neurotransmitters related to pain signaling. We will discuss this in more detail in the section on opioid pharmacodynamics later in this chapter.
12.2. Pharmacokinetics
Section Learning Objectives
- Explain the different ways opioids can be administered and what properties influence the subjective experience of the drug.
- Describe the metabolism of various opioids, in particular morphine, heroin, and codeine, and explain how they are related.
- Explain the low bioavailability of orally administered morphine.
In this section, we will discuss the pharmacokinetics of opioids. Because morphine is the prototypical opioid, we will use its pharmacological properties as a baseline. When there are notable differences in how other opioids behave, we will highlight the differences and compare them.
12.2.1. Absorption and Distribution
Opioids can be administered in myriad ways. Although they are most often injected or taken orally, opioids can also be administered sublingually, subcutaneously, intranasally, rectally, epidurally, buccally, or inhaled. The route of administration is usually determined by the reason for use. Opioid pain medications like oxycodone often come in pill or tablet forms, which are convenient for outpatient settings but have low bioavailability because of the first-pass effect. For this reason, morphine is typically injected intravenously (i.v.) or intramuscularly (i.m.) in hospitals.
Illicit use of opioids also follows the same pattern. Because IV administration results in the fastest onset of symptoms, injection is often preferred. Another common method is to vaporize heroin or other opioids by heating them and inhaling the smoke, a process known as “chasing the dragon.”
In general, opioid administration causes a four-phase subjective effect. The first phase is the rush or rapid onset of euphoria that occurs seconds after injection. This is followed by the high, which causes a feeling of joy and ease. The third phase is called the nod and is characterized by feelings of calm and disinterest. During this phase, the user may feel no anxiety and engage in light sleep. The final phase is the straight phase, which is a period of normalcy (Agar, 1974).
- Rush = sensation of heroin entering system
- High = pleasurable state, chemically induced
- Nod = semi- or unconscious state induced by heroin
- Straight = not sick
Once absorbed, opioids are capable of crossing the blood-brain barrier. Because different opioids have different lipid solubilities, some are absorbed and cross the blood-brain barrier faster than others. A prime example is heroin, which has greater lipid solubility than morphine, which is why it has a faster onset of action. Peak concentrations of morphine occur in about 20 minutes, while heroin can peak in 5 minutes.
The onset of action is a large contributor to which opioids have the highest potential for addiction. Certain synthetic opioids, such as fentanyl and its derivatives, have a rapid onset and a potency hundreds of times greater than morphine. Recall the opioid epidemic graph shown in the previous section. Prescription opioids, which are taken orally, tend to have a slower rate of absorption and longer onset of action. As users switched to heroin and fentanyl, the rush of euphoria increased as did the potency, creating a stronger dependence on the drug and increasing the risk of overdose.
12.2.2. Metabolism and Excretion
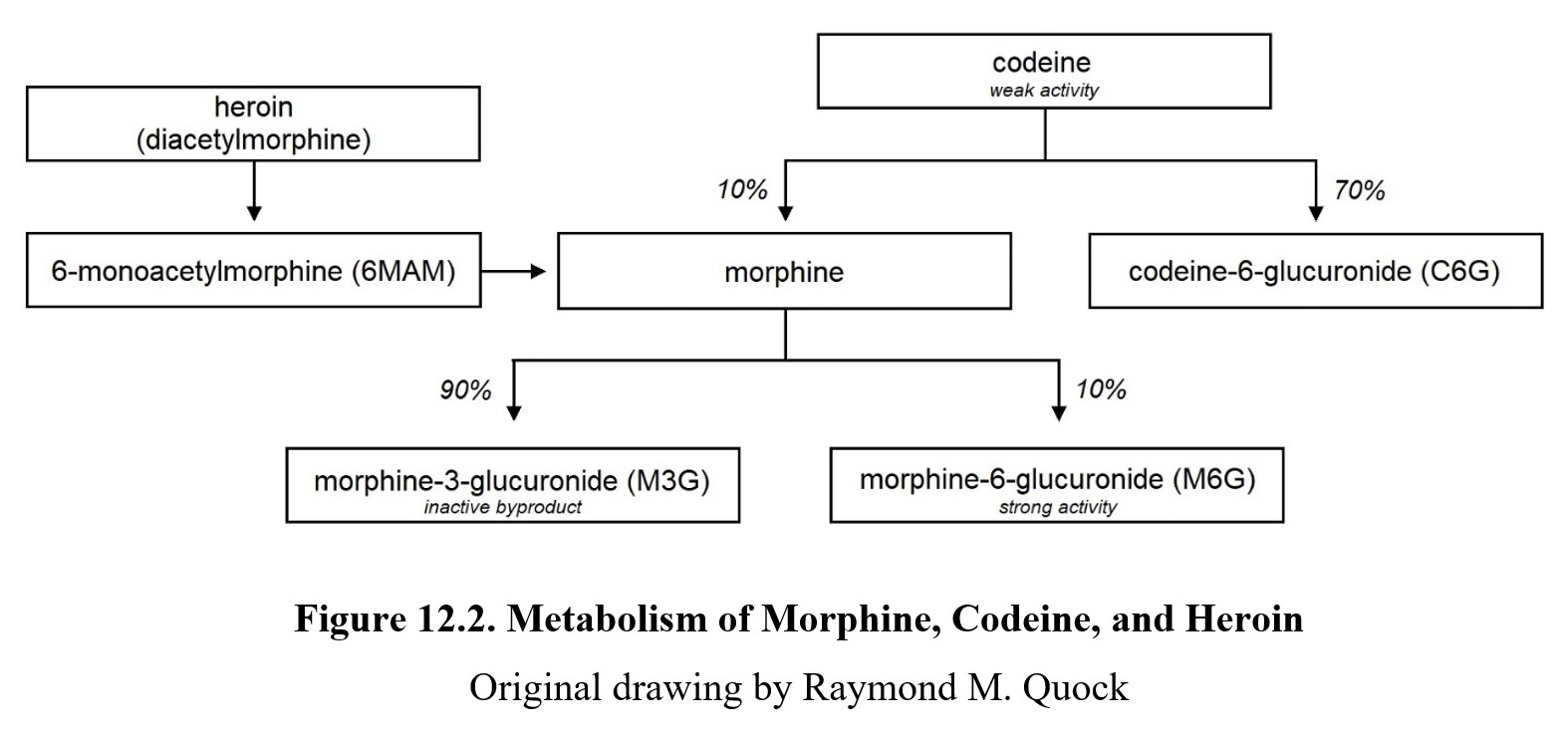
Metabolism of morphine and other opioids largely takes place in the liver. Morphine is metabolized by linking it with glucuronic acid to form a glucuronide conjugate. About 90% is converted to morphine-3-glucuronide (M3G), while the remaining 10% is converted to morphine-6-glucuronide (M6G). MG3 is inactive, while MG6 is active and produces a powerful analgesic effect. Glucuronides are easily eliminated through urine and feces. Morphine typically has a half-life of around 2 hours, although there can be individual differences in the rate of metabolism and excretion.
Because semisynthetic opioids are derivatives of natural opioids, many are metabolized into other opioids. Heroin, or diacetylmorphine, is a prodrug that enters the body and becomes activated by losing the acetyl groups. It is first converted to 6-monoacetylmorphine, which is, in turn, metabolized into morphine. Another example is codeine. Codeine is also converted directly to morphine, similar to heroin. You have seen this pattern before—when we discussed stimulants, we learned that methamphetamine is converted into amphetamine, which is part of the reason why the former is so much stronger.
So why isn’t codeine stronger than morphine? The answer is that codeine is only partially converted into morphine (about 5–10%). Most of the codeine is metabolized into codeine-6-glucuronide (C6G), which is active and eliminated readily. Codeine itself is much less pharmacologically active and acts more like a prodrug for morphine and C6G.
The diagram below shows the metabolic relationships described above. (Note that there are other metabolites not included in this diagram.)
One particular opioid worth mentioning is methadone. We have mentioned methadone before as a treatment drug used in opioid replacement therapy. One of the reasons why methadone is so effective as a treatment drug is because it has a much longer half-life than most opioids. In someone with opioid tolerance, the half-life is around 24 hours. In patients without tolerance, this increases to 55 hours (Grissinger, 2011). This means that methadone is active for longer and can be administered less frequently, usually in an oral daily dose.
12.3. Pharmacodynamics
Section Learning Objectives
- Explain the pain system and describe how morphine and other opioids influence pain transmission and perception.
- Describe the various effects of morphine on the body besides pain relief.
- Describe the differential development of tolerance to the effects of opioid drugs.
- Describe the cardinal signs of opioid overdose
- Explain the emergency treatment of opioid overdose.
- Describe opioid dependence and withdrawal.
- Describe various treatment approaches to opioid use disorder.
In this last portion of the chapter, we will explore the various ways that opioids influence physiological functioning. Similar to the previous section, we will primarily be discussing the mechanism of action for morphine. Most opioids have similar effects as morphine, though there are some differences. Keep this in mind as you move forward.
12.3.1. Pain Relief
Morphine’s main site of action is the mu-opioid receptor. Its affinity and efficacy are highest for this receptor subtype, although it is also an agonist at the delta- and κ-opioid receptors. Activation of the MOR is the primary cause of the analgesic effect of opioids.
To understand how opioids like morphine provide pain relief, it is necessary to first understand how pain works. Nociception is the sensation of pain stimuli and begins in nociceptors, which can be found throughout the body in the peripheral nervous system. Nociceptors convert potentially damaging mechanical, chemical, or thermal stimuli into electrical signals, a process known as transduction.
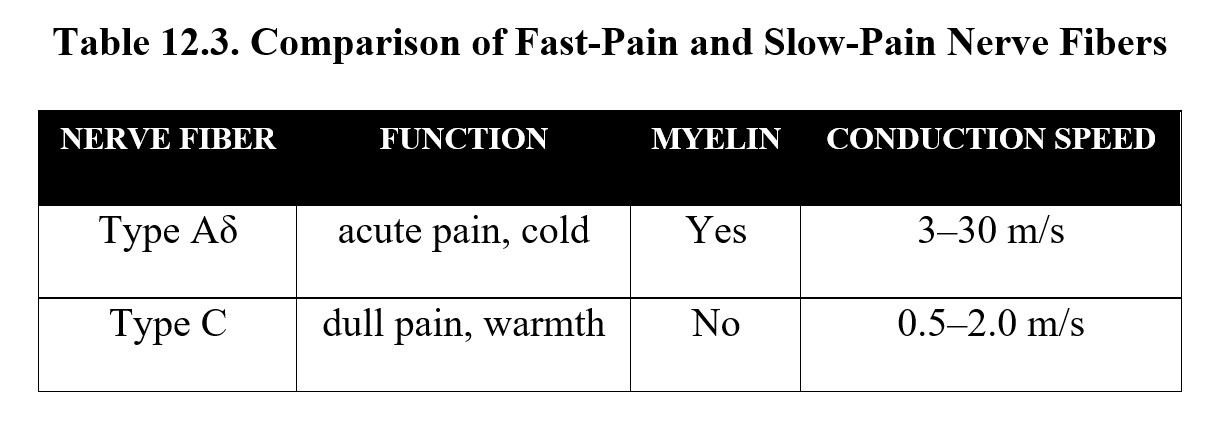
Once converted, the signal must be carried along axons to the CNS through transmission. If the axons are myelinated, the signal propagates quickly and results in acute, sharp pain. These are known as Type Aδ nerve fibers. Type C fibers, on the other hand, are unmyelinated and transmit pain signals more slowly, resulting in dull, aching pain. This is the explanation for fast pain and slow pain after sustaining an injury. There are other types of sensory nerve fibers, but these two are the main types involved in nociception. Compare the two types below:
The sensory afferent fibers travel from the nociceptor to the spinal cord, where the pain signals are then carried along the ascending spinothalamic pathway to the brain. Specifically, signals are routed through the thalamus to the somatosensory cortex, which is responsible for pain perception, or processing and interpreting the pain signal. Once the brain is aware of the pain, it will attempt to regulate it through modulation. The periaqueductal gray (PAG) and rostral ventromedial medulla (RVM) send descending signals to inhibit the incoming pain signal at the spinal cord. See the image below for the entire process:
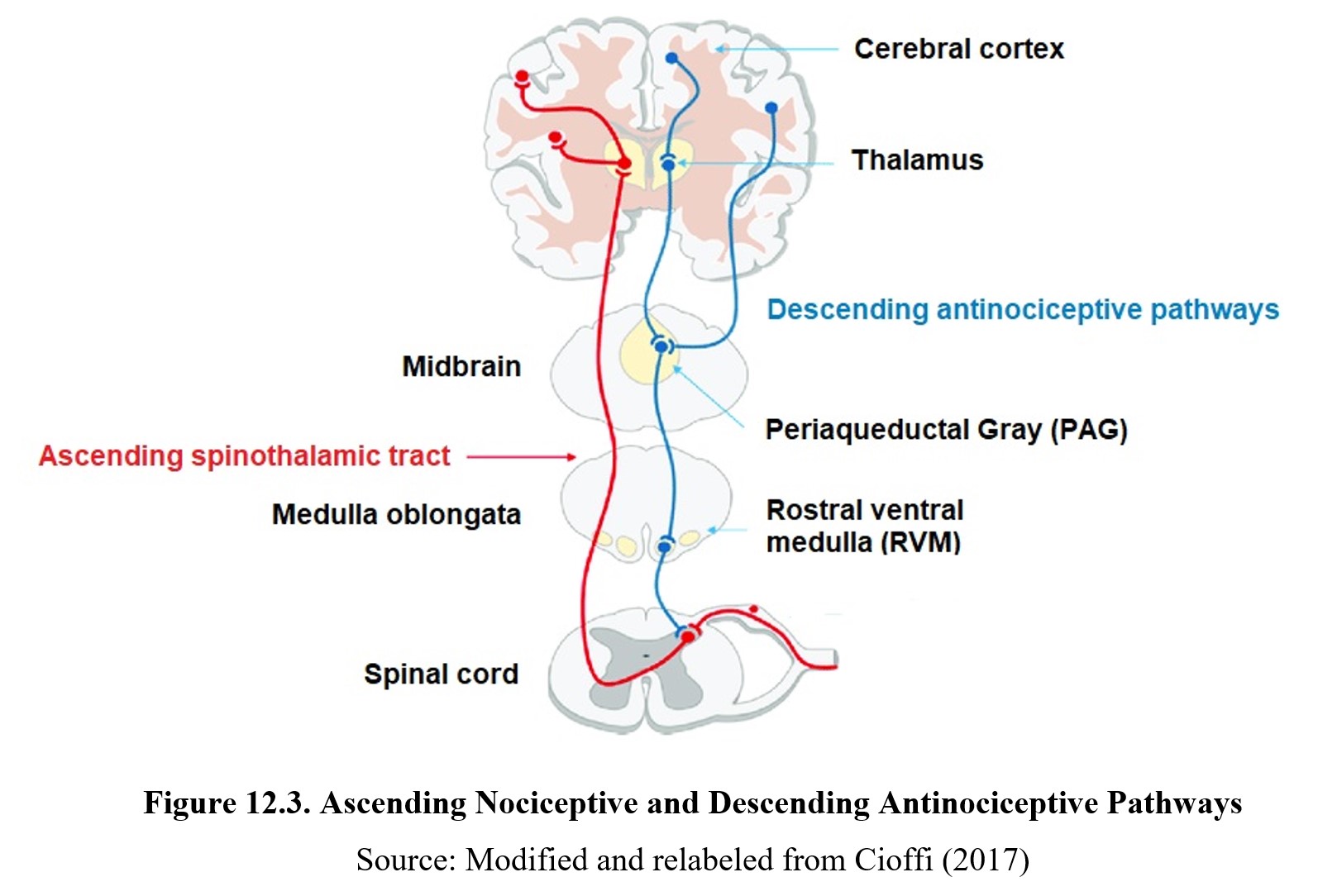
By way of review, pain occurs in four stages: transduction, transmission, perception, and modulation. Transduction and transmission occur in nociceptors and afferent sensory nerve fibers, which can either be myelinated or unmyelinated. Pain signals ascend from the spinal cord to the thalamus then to other brain receptors for perception, while inhibitory signals descend from the PAG and RVM to the spinal cord, resulting in modulation. Some of these descending antinociceptive pathways are opioid and cause the release of endorphins. Norepinephrine and serotonin have also been implicated in descending pain-modulating pathways.
How do opioids affect pain signals? Recall that opioid receptors can be both presynaptic and post-synaptic. Presynaptic opioid receptors are located on the axon terminals of the Aδ and C fibers. The axons of these first-order afferent neurons synapse in the dorsal horn of the spinal cord and release glutamate and Substance P when an action potential or pain signal arrives. Activation of postsynaptic glutamate- and Substance P-sensitive receptors on the second-order afferent neuron recreate the pain signal in the ascending pathway. Endorphins can activate presynaptic opioid receptors to reduce or inhibit the release of glutamate and Substance P, which diminishes pain transmission. Endorphins can also activate postsynaptic opioid receptors to decrease the strength of the depolarization initiated by glutamate and Substance P. The combined effect of presynaptic and postsynaptic opioid receptor activation is to diminish the pain signal headed for the brain.
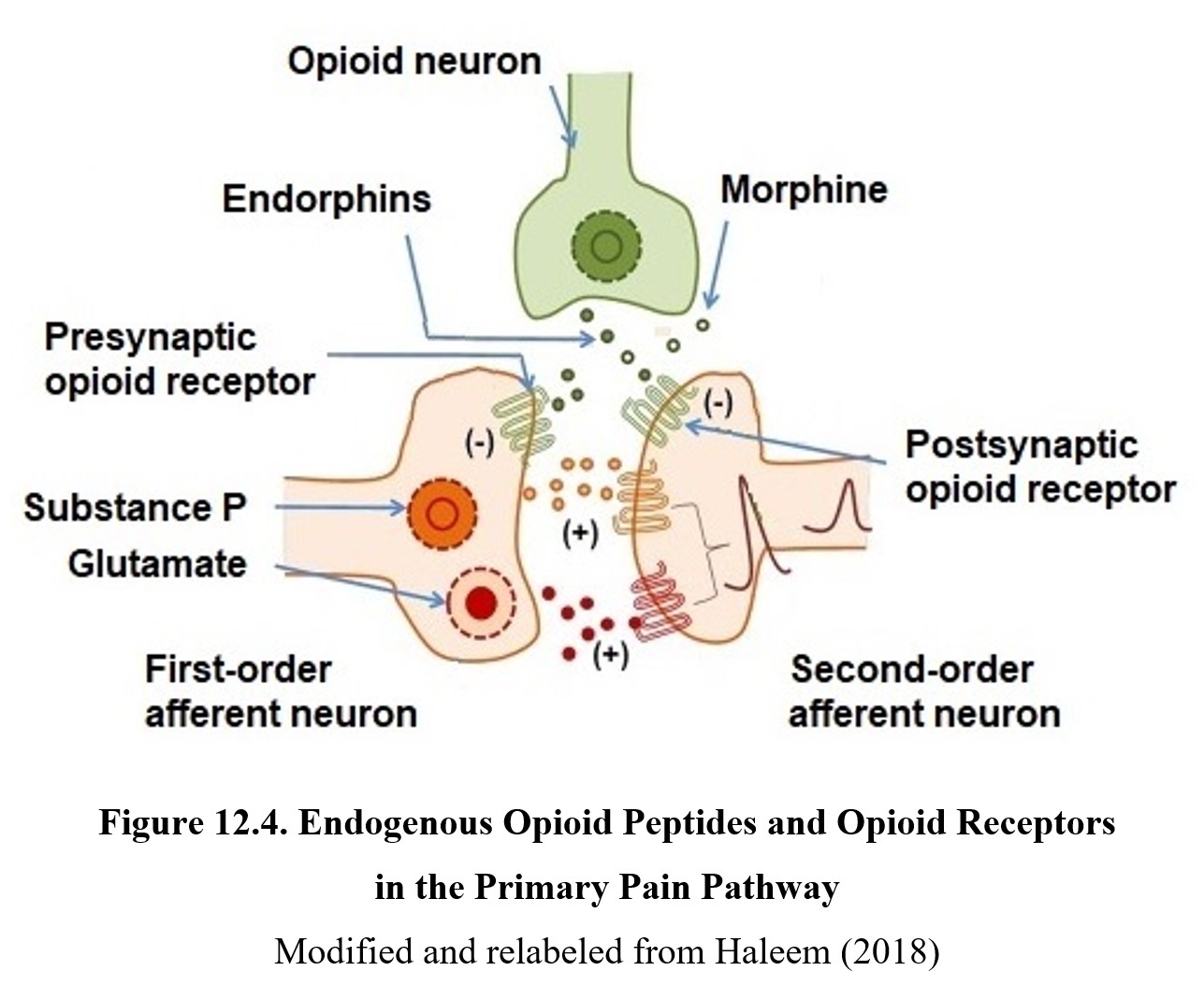
When the original pain stimulus is so intense that activation of the endogenous opioid system is insufficient to mitigate the pain, exogenous opioid drugs may be administered to the patient to control the pain. Exogenous opioids will similarly activate presynaptic and postsynaptic opioid receptors to moderate the pain signal.
Opioids are best suited for reducing chronic dull pain since they have a greater inhibitory effect on Type C fibers than the Type Aδ fibers responsible for acute pain. They are less effective for neuropathic pain, which is caused by nerve damage. Despite this, at high enough doses, opioids will reduce almost any type of pain. Because they are highly effective but also addictive, opioids are usually used to treat moderate-to-severe pain.
Opioid Mechanism of Action [5:15]
12.3.2. Other Effects
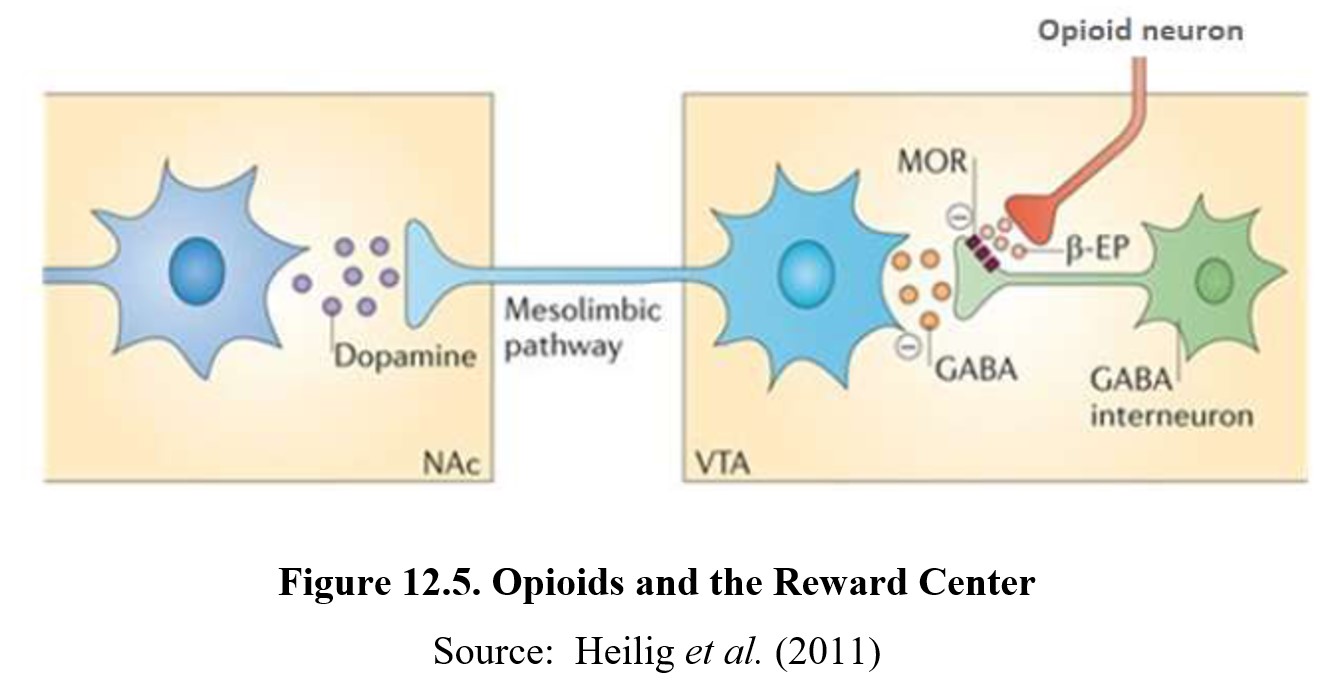
Considering how opioid dependence has been firmly established as a potential danger of opioid use throughout this chapter, it should come as no surprise that opioids increase dopamine transmission in the reward system. In Chapter 11, we saw how ethanol causes neuronal release of beta-endorphin (β-EP) to activate mu opioid receptors (MOR) on a GABA interneuron. The opioid receptors are inhibitory and will reduce the release of GABA from the interneuron. Freed from GABA inhibition, the VTA neuron releases dopamine to activate its receptors in the nucleus accumbens (NAc). The mechanism is very similar to how opioids act upon the reward center except that morphine directly activates the opioid receptors rather than releasing an endorphin. Activation of mu opioid receptors (MOR) on GABA interneurons in the ventral tegmental area (VTA) and decreases the tonic GABA inhibition of the dopamine neuron. The disinhibited VTA neuron thus increases its release of dopamine in the nucleus accumbens:
Opioids produce a myriad of effects on the body. Opioid receptors are widely distributed body and have effects on virtually every physiological and psychological function. If we examine the effects of oxycodone, a popular prescription painkiller, we find that its effects extend beyond control of pain.
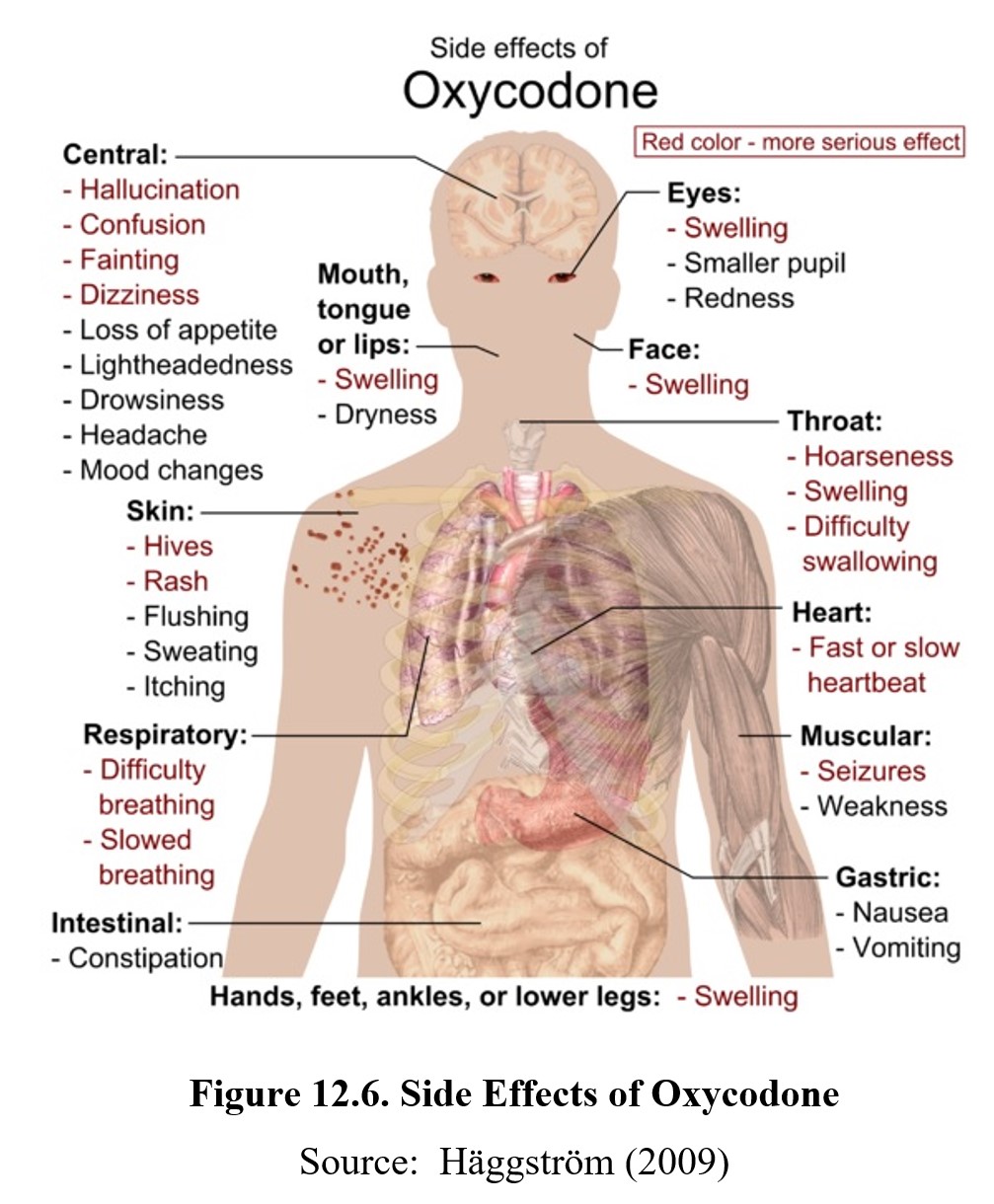
Because opioid receptors are found throughout the nervous system, opioids cause other effects aside from pain relief. In the CNS, effects include sedation and euphoria. Opioids can interact with other CNS depressant drugs to produce a synergistic degree of depression.
Recall from earlier that heroin was originally marketed as a cough suppressant. Indeed, opioids, especially codeine and hydrocodone, exhibit powerful antitussive (cough-preventing) properties, which is why they can be found in some cough syrups and cold medications. The cough reflex is managed by the cough center in the medulla and is triggered in response to irritation in the lungs and airways. Opioids reduce coughing by increasing the threshold for the cough reflex in the medulla. This antitussive effect of opioids is not mediated by opioid receptors. The popular antitussive drug dextromethorphan is the dextro enantiomer derived from a levo opioid drug; it has poor affinity for the mu-opioid receptor yet retains the antitussive property.
Opioids also have marked effects on respiration. Breathing is an involuntary action that is also stimulated by various chemical cues. First, there is a respiratory drive, in which chemoreceptors in the medulla oblongata are activated by the detection of large amounts of carbon dioxide in the blood. And second, there is also a hypoxic drive, in which chemoreceptors in the carotid artery and ascending aorta are activated by the detection of low amounts of oxygen in the blood. Morphine and other opioids specifically act on mu-opioid receptors in the respiratory center to reduce the responsiveness of the chemoreceptors to high levels of carbon dioxide, resulting in respiratory depression and, in severe cases, failure.
Another process in the medulla that is influenced by opioids is nausea and vomiting. Opioid activation of delta- and kappa-opioid receptors cause stimulation of the chemoreceptor trigger zone (CTZ) which, in turn, activates the emetic or vomiting center to induce nausea and vomiting. Opioids also activate mu-opioid receptors in the vomiting center to cause a delayed inhibition of activity and reduction in nausea and vomiting. In other words, initial doses of an opioid painkiller cause vomiting, but with repeated doses, nausea decreases. This is why people often feel nauseous after their surgical procedure where opioids are used to suppress pain. But they often feel less nauseous with subsequent doses. This is not the development of tolerance but is due to the time difference in the actions of opioids on the two medullary centers.
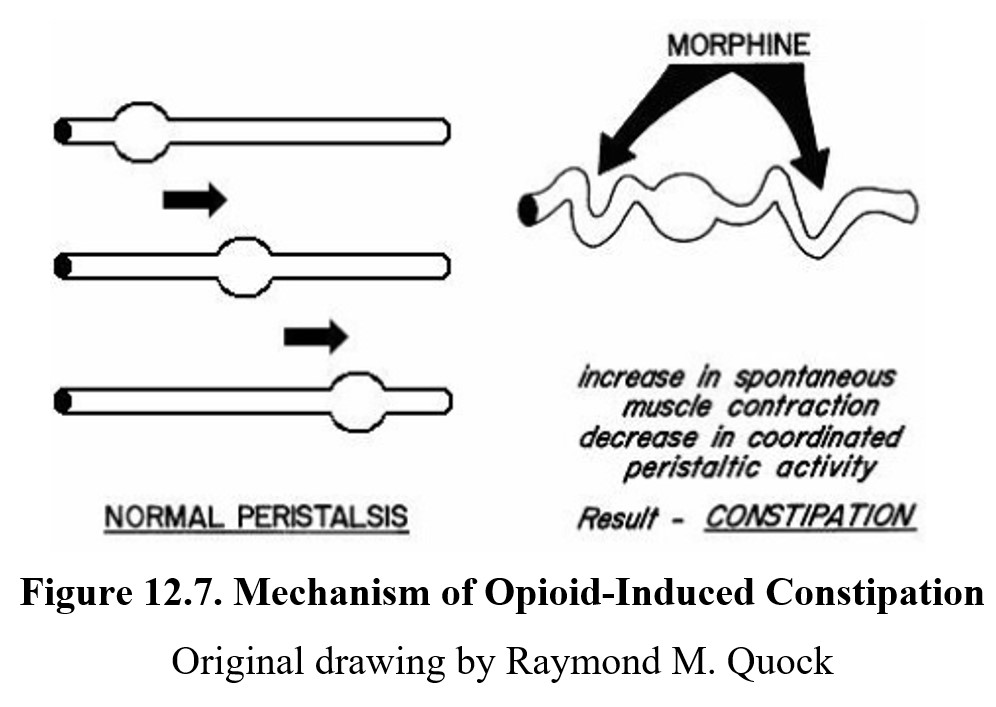
Another frequent side effect associated with opioid use is constipation. Normally, the smooth muscle in the intestines moves food through the tract through coordinated contraction and relaxation of the smooth muscle, known as peristalsis. Opioid receptors in the gastrointestinal tract are excitatory and can cause spontaneous contractions of the muscle. This reduces peristalsis and results in constipation (Fig. 12.7). It is important to know that even with chronic drug treatment, there is no development of tolerance to the constipating effect of opioids.
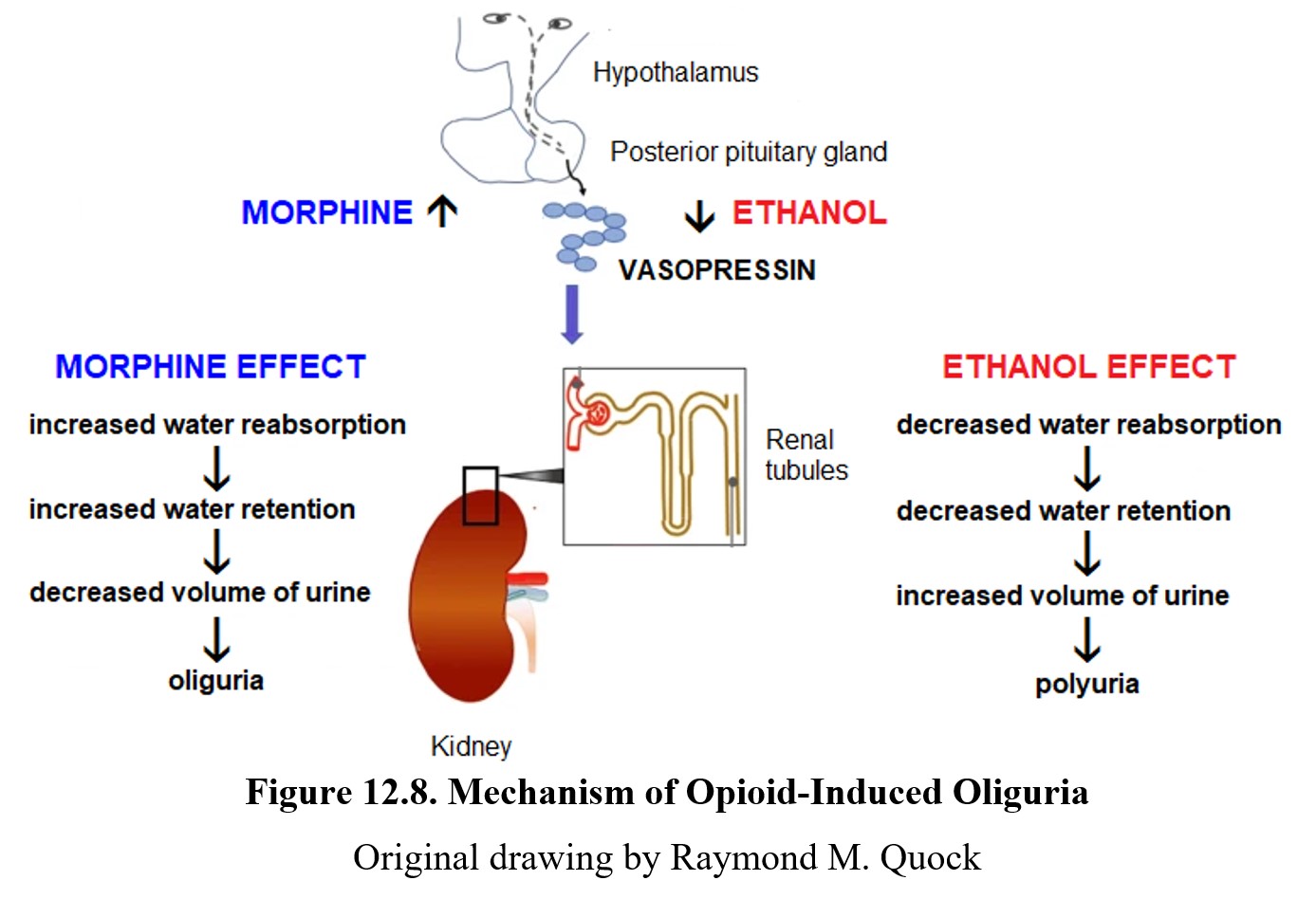
Opioids cause a variety of other effects. In the kidneys, opioids stimulate the secretion of vasopressin, which promotes the reabsorption of water from the kidneys and results in a decreased volume of urine known as oliguria. Note that this is the exact opposite effect from ethanol which reduces the reabsorption of water and increases the production of urine (polyuria).
Activation of opioid receptors in the oculomotor nerve also causes pupillary constriction, often so extreme that it is referred to as pinpoint pupils. This is another opioid drug effect to which there is no development of tolerance upon repeated drug use. This is an unavoidable sign to law enforcement officials of recent opioid use.
Opioids also inhibit gonadotropin release which results in hypogonadism, a condition characterized by decreased testicular size and erectile dysfunction in men, also infertility, missed periods, and hot flashes in women. Finally, exogenous opioids are also implicated in immunosuppression.
12.3.3. Tolerance and Overdose
As noted above, tolerance develops to the effects of morphine except for constipation and pupillary constriction. Not all effects develop tolerance at the same rate, however. Tolerance to pain relief and euphoria tends to develop at lower doses than respiratory depression (see image below). As users will typically escalate opioid use to achieve similar effects, long-term users will often begin to develop a tolerance for respiratory depression as well. This is unlike the pattern of tolerance to euphoria and respiratory depression during continuous use of CNS depressants such as barbiturates (Chapter 10).
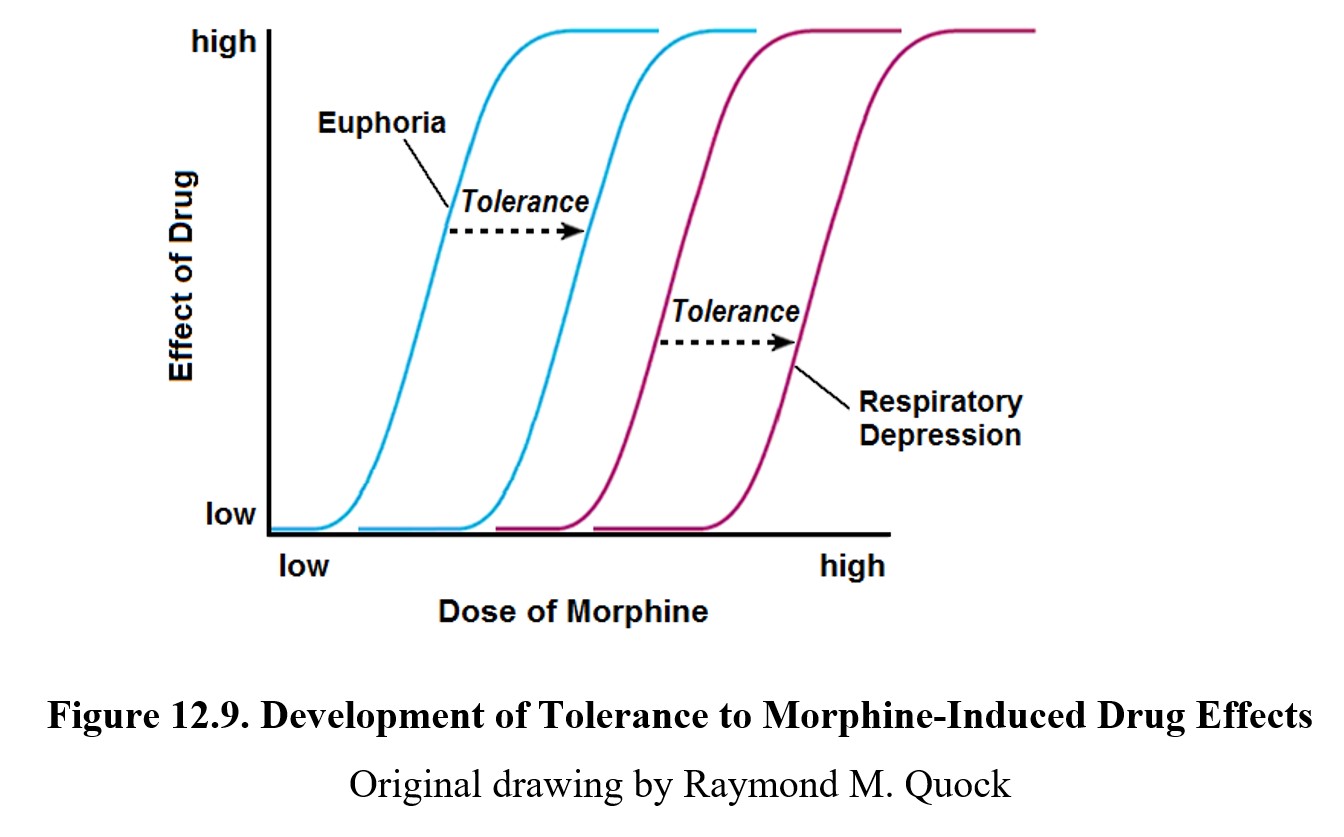
Because opioids share similar mechanisms of action, there is a high degree of cross-tolerance between different opioids. This is why during the opioid epidemic, users of prescription opioids could transition to more potent drugs such as heroin and fentanyl.
Although tolerance to respiratory depression can be built, it is highly context-dependent and involves a conditioned compensatory response. Recall from Chapter 7 that this is an automatic response that is learned through repeated drug use. Cues in the environment such as location, time of day, or the presence of other people signal the body to prepare for the effects of the drug. This is why administering the same dose in a different setting can result in an accidental overdose.
Opioid overdose is severe and comes with many signs, most of which mimic the typical effects of the drug. Respiratory depression, pinpoint pupils, stomach and intestine spasms, drowsiness, disorientation, and loss of consciousness are all hallmark signs, as are dry mouth, low blood pressure, and bluish-colored nails and lips. When opioids are combined with other depressants, respiratory depression is intensified. Most deaths due to overdose are caused by respiratory failure.
To reverse opioid overdose, an opioid antagonist such as naloxone or naltrexone is administered. These drugs have a high affinity for opioid receptors and bind to them, displacing the opioid and reversing its effects. Opioid antagonists are commonly injected, although a naloxone nasal spray has been developed and approved by the FDA for emergency overdose treatment. Nasal sprays are easier to use and reduce the risk of emergency responders contracting blood-borne diseases.
Different opioids have different rates of overdose. An overdose on fentanyl, a potent synthetic opioid, can occur very quickly, which reduces the window for emergency treatment. This is part of the reason why fentanyl and fentanyl derivatives have been singled out as particularly dangerous drugs responsible for many deaths during the recent opioid epidemic, especially since their use has increased in the past few years.
12.3.4. Opioid Use Disorder
Misuse and abuse of opioid drugs can result in opioid use disorder which is characterized by an intense craving for an inability to abstain from taking opioid drugs. There is an obsession with how to obtain the “next fix” at the expense of other obligations to family, friends, and work. The U.S. is currently in the midst of an opioid epidemic as indicated in the figure below:
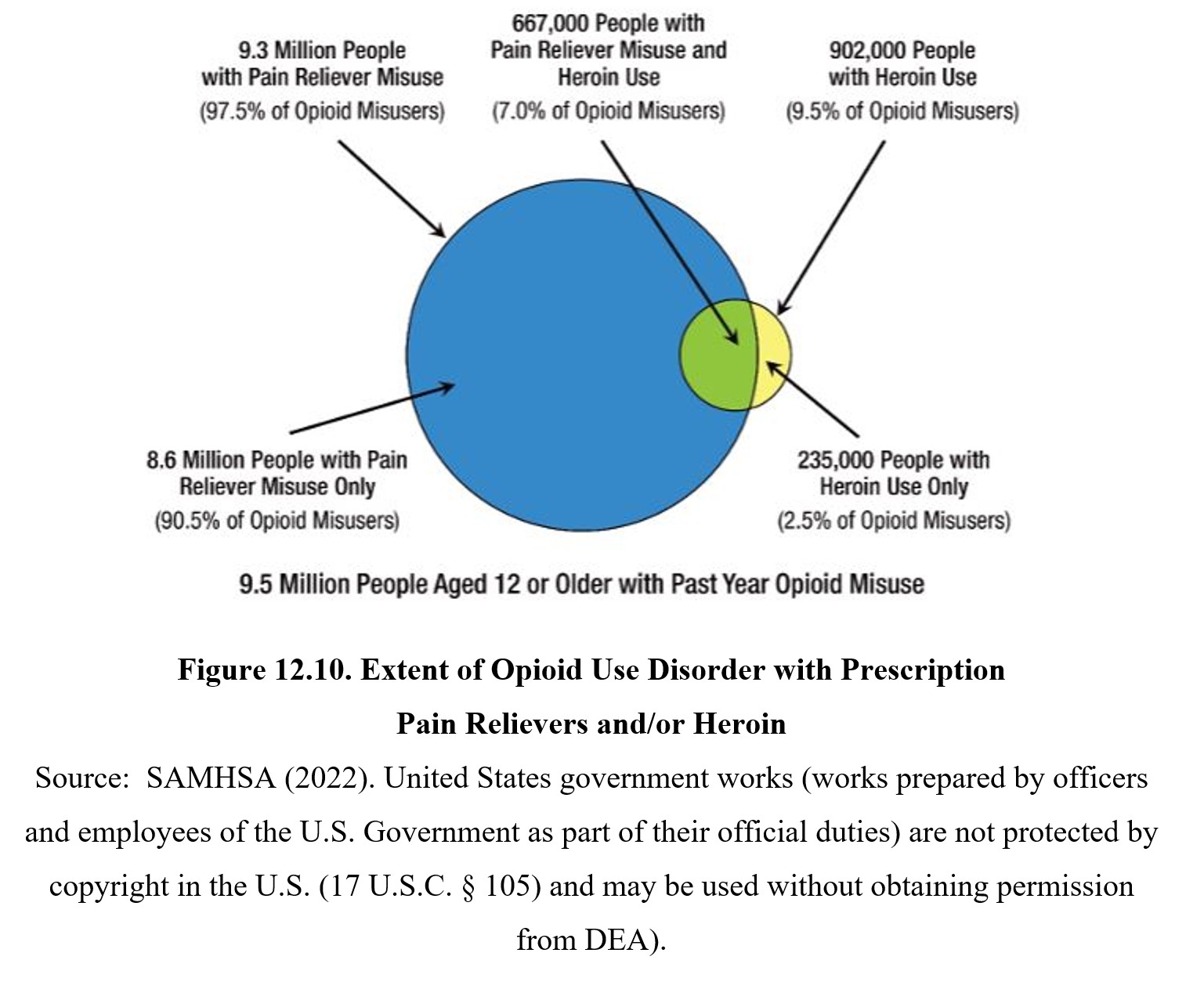
12.3.5. Dependence, Withdrawal, and Treatment
Similar to dependence exhibited with other drugs, opioid dependence is characterized by an overwhelming need to acquire and use an opioid drug. Addiction is easily developed as compulsive drug use will persist despite negative consequences. Opioid dependence is common and once established, can be difficult to escape. Relapse is common. Heroin is the most notorious of abused opioids, although fentanyl derivates have likely surpassed it.
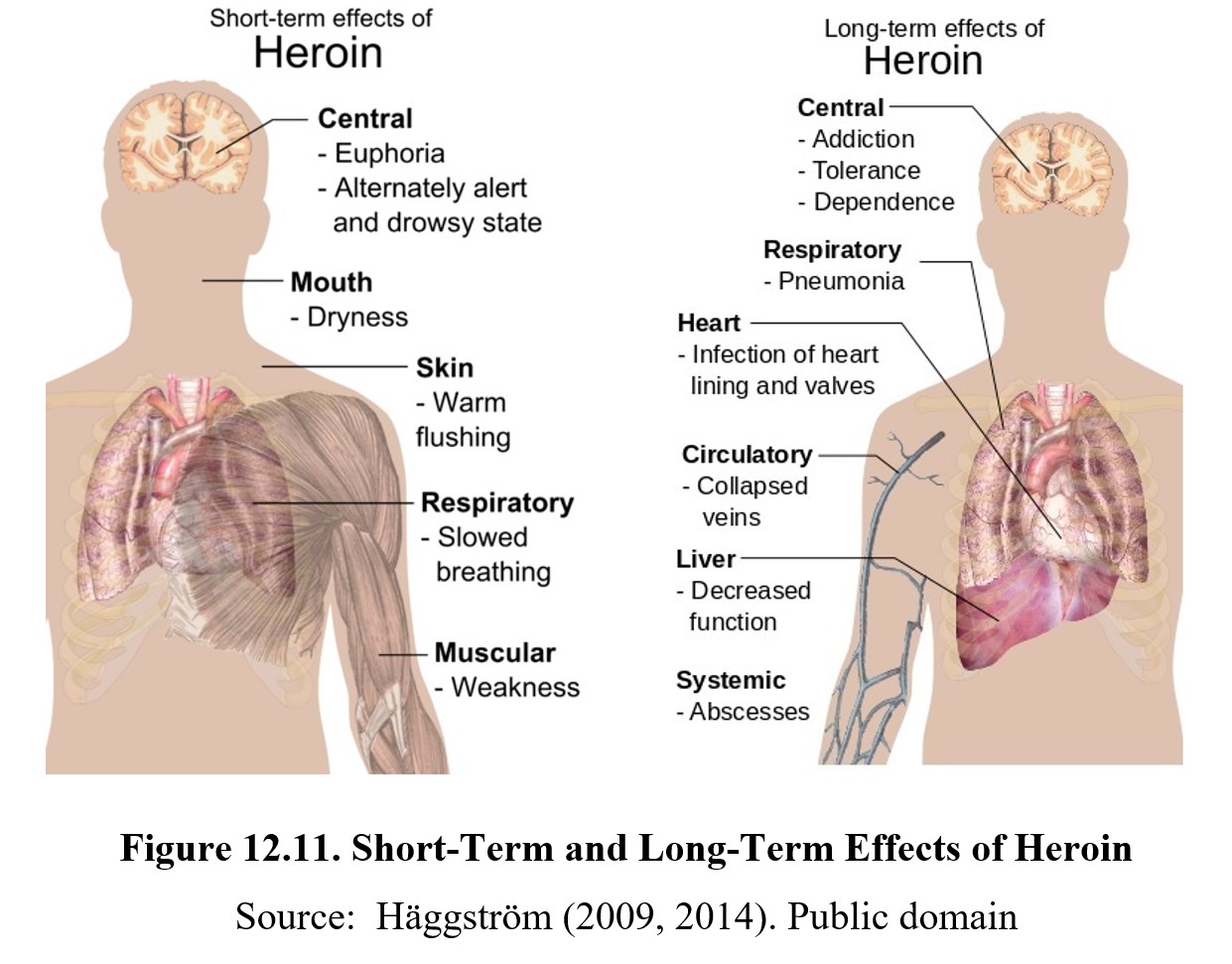
Heroin has a relapse rate of 50-85%, and chronic users have been known to report cravings years after treatment. One of the reasons why opioid dependence is so strong is because withdrawal symptoms are unpleasant and often severe. As is typical, withdrawal symptoms are the inverse of drug effects. The most notable symptom is hyperalgesia or increased pain sensitivity. This is caused by the pain system compensating for the inhibitory effects of opioids. With chronic use, this can result in opioid-induced hyperalgesia, where opioid pain medications increase pain instead of providing relief.
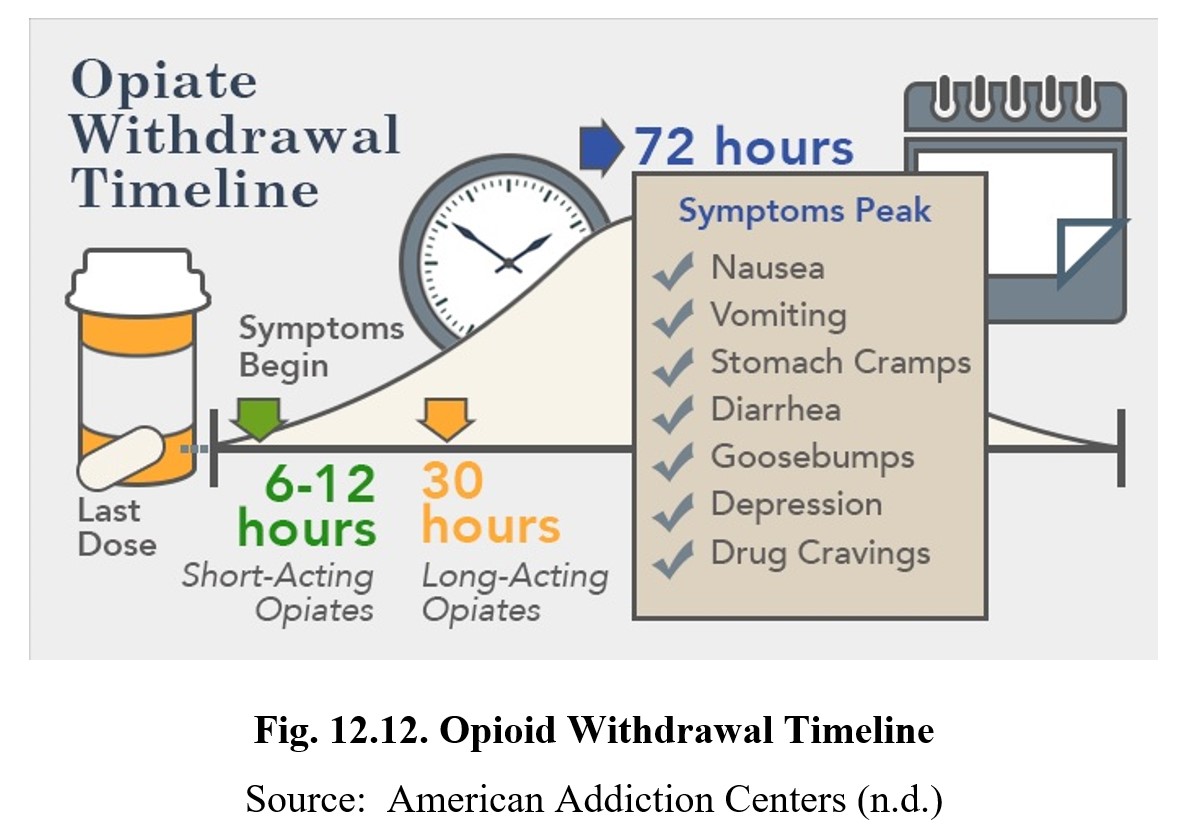
Withdrawal symptoms also include dilated pupils, sweating, nausea and vomiting, diarrhea, aches and pains, low blood pressure and heart rate, insomnia, anxiety, hyperactivity, and depression. These symptoms occur at different times and can last for weeks or months, although peak withdrawal symptoms occur within the first 72 hours (see the chart below). An exception to this is precipitated withdrawal or withdrawal caused by the administration of an opioid antagonist such as naloxone. In this case, maximum withdrawal can occur within minutes.
What Causes Opioid Addiction, and Why Is It So Tough to Combat? [8:21]
There are numerous methods for combating opioid dependence. One form of prevention strategy is to reformulate prescription opioids to make them harder to misuse. In the wake of the opioid epidemic, the manufacturers of OxyContin® changed the dosage form to be harder to crush or dissolve. Most approaches involve treatment and relapse prevention, which can be done through a combination of drug therapies and psychotherapies.
Psychotherapy is always required for the treatment of opioid use disorder. Dependence is more than just physical dependence. There remain potent psychological and social triggers that can increase the risk of relapse. These are best addressed by cognitive-behavioral therapy, contingency management, and family counseling.
The most common form of drug therapy for opioid use disorder is drug replacement, which involves administering a safer opioid with weaker effects to mitigate withdrawal. One such drug that we mentioned earlier in this chapter is methadone (Dolophine®). It is slower-acting and longer-lasting compared to heroin and morphine and can be administered once a day. Methadone does not cause a rush, nor does it cause drowsiness or impairment of thinking, emotions, or sensations. Despite this, there is still some potential for misuse, as higher doses can reproduce the desired effects.
An alternative to methadone is buprenorphine (Buprenex®). Compared to methadone which is a full opioid agonist, buprenorphine is a partial agonist. This means it has a lower potential for misuse while still being effective at mitigating withdrawal symptoms.
Another example is Suboxone®, a combination of buprenorphine and naloxone. If taken orally, the naloxone has poor bioavailability due to first-pass metabolism and has no effect. However, if crushed and injected, the naloxone will block opioid receptors and precipitate withdrawal in patients with opioid dependence.
If the patient is already opioid-free and wants to prevent relapse, naltrexone (Vivitrol®) is another option. As mentioned before, naltrexone is an opioid antagonist, which is why it cannot be administered to patients who have recently used opioids. Otherwise, it will precipitate withdrawal. Naltrexone is orally active and can be taken daily as a pill (ReVia®) or injected once a month (Vivitrol®). Because it is an antagonist, it is not used to mitigate withdrawal symptoms. Instead, it prevents the pleasurable effects of future opioid use. Compared to methadone or buprenorphine drug replacements, there is no risk of misuse.
Drug therapies are usually paired with some form of psychotherapy because even with the treatment of physical dependence, there are still psychological cues and triggers that can induce relapse. Cognitive-behavioral therapy (CBT) can reduce cravings and provide the patient with coping strategies to reduce the need for drugs. Contingency management programs reward opioid abstinence through reinforcement with vouchers or other redeemable prizes. Finally, family counseling is often used to help educate friends and families about opioid use disorder and provide a social support network.
Chapter Summary and Review
In this chapter, we explored opioids such as morphine, heroin, oxycodone, and fentanyl. We started by defining the three classes of opioids and the endogenous opioid system, while also covering the history of opioid use and the recent opioid epidemic in the U.S. We then discussed the pharmacokinetics of morphine and compared it to other opioids. Finally, we learned about the pain system of opioid use and explored the other effects of opioids, as well as opioid tolerance, dependence, overdose, and treatment.
Chapter 12 Practice Questions
Answer the following questions:
- Define natural, semisynthetic, and synthetic opioids and provide two examples of each.
- Describe the recent opioid epidemic. What drugs were implicated, and how has the epidemic changed over the years?
- Name the three subtypes of opioid receptors. Which endogenous opioid peptides have selective affinity for each?
- Why is morphine usually injected in hospital settings?
- Explain how codeine is metabolized and why its metabolites are so important.
- What are the two main metabolites of morphine? Describe their proportions and whether they are active or inactive.
- Explain the difference between Type Aδ and Type C nerve fibers.
- What two areas are the origin of descending inhibitory signals that moderate pain?
- Name five effects of morphine besides pain relief.
- Name four signs of opioid overdose. What drug can be used for emergency treatment?
- When do peak withdrawal symptoms occur during natural withdrawal? What about precipitated withdrawal?
- Describe three different drug therapies for the treatment of opioid dependency and how they work.
2nd edition
