Chapter 16: Antidepressants
2nd edition as of August 2022
Chapter Overview
In this last unit of the course, we will be covering psychotherapeutic drugs that are used to treat mental disorders and physiological conditions that cause behavioral, cognitive, and personality changes. We will start with the affective disorders and the drugs used to treat them.
Chapter Outline
- 16.1.1. Types of Mood Disorders
- 16.1.2. Monoamine Hypothesis of Depression
- 16.1.3. Pharmacological Treatment
- 16.1.4. Non-Pharmacological Treatments
- 16.2.1. Overview of Antidepressants
- 16.2.2. Monoamine Oxidase Inhibitors
- 16.2.3. Tricyclic Antidepressants
- 16.2.4. Selective Serotonin Reuptake Inhibitors
- 16.2.5. Other Atypical Antidepressants
- 16.3.1. Symptoms of Bipolar Disorder
- 16.3.2. Mood Stabilizers
Chapter Learning Outcomes
- Outline types of mood disorders, causes, and treatment options.
- List and describe types of antidepressant drugs.
- Describe the symptoms of bipolar disorder and outline how mood stabilizers are used to treat it.
16.1. Depression
Section Learning Objectives
- Define the major types of mood disorders.
- Differentiate between the diagnostic criteria of major depressive disorder and persistent depressive disorder.
- Describe the neurochemical causes of mental depression.
- Differentiate between depressions caused by different chemical imbalances.
- Describe the goal of pharmacological treatment of depression.
- Provide two possible explanations for the delayed effect of antidepressants.
- Discuss the use of psychotherapy and electroconvulsive therapy in the treatment of depression.
In a clinical setting, mental depression refers to a negative change in mood that is more severe or persistent than normal. In this section, we will define depression and explore some of its causes and treatments.
16.1.1. Types of Mood Disorders
Affective disorders (from affectus in Latin for “mood”) refer to mood dysregulation. It is normal for people to have shifts in mood. At times, people may even dip into moments of depression, such as after the loss of a job or relationship, or moments of heightened excitement and activity, known as mania. These changes are usually mild though and do not last for long periods. However, disorders occur when mood changes are severe or persistent and exceed the limits of normal or balanced mood.
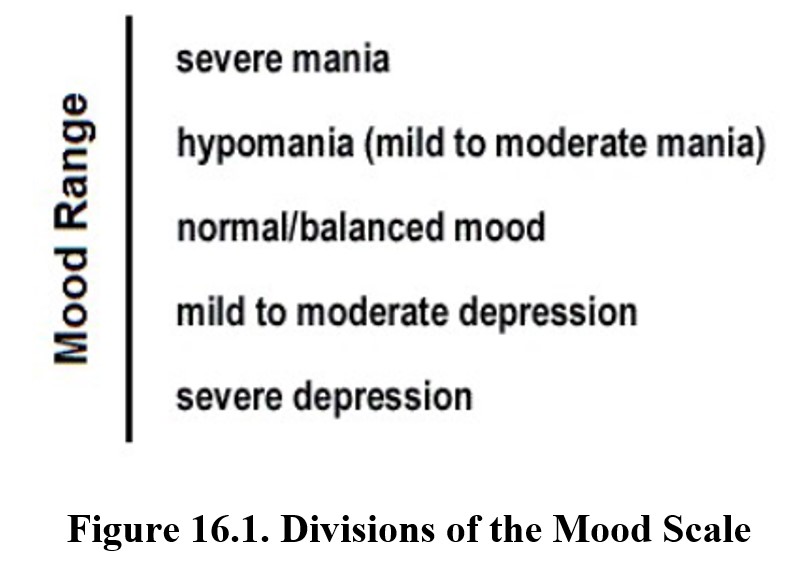
There are two primary types of affective disorders: depression and bipolar disorder. Bipolar disorder may include varying degrees of mania, which we will discuss in detail in a later section. For now, we will focus on depression, of which there are two major forms.
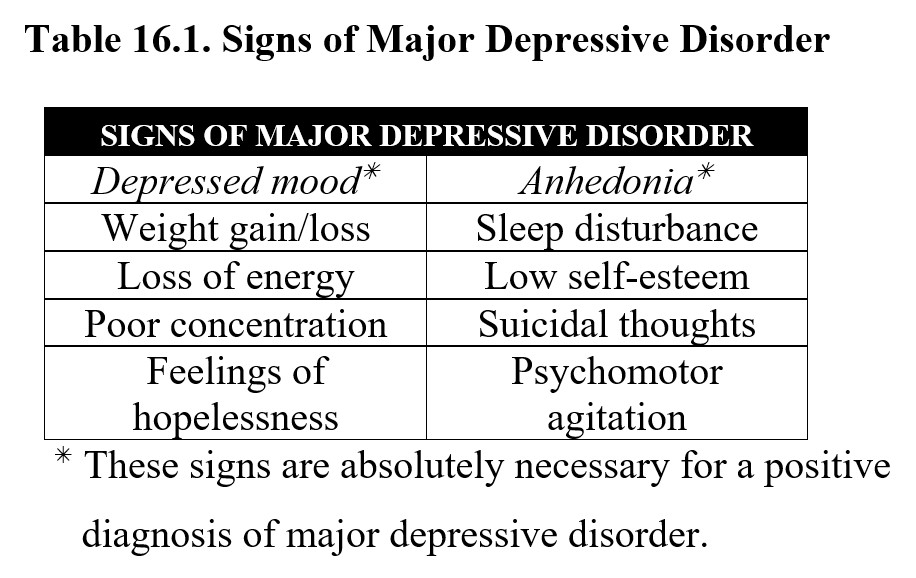
The first is major depressive disorder or MDD. The critical symptoms of MDD are depressed mood and anhedonia, or a loss of interest or pleasure. These symptoms must last for at least two weeks and be accompanied by at least three additional symptoms. Consult the following table for a full list, but note that the most important symptoms are the two described above:
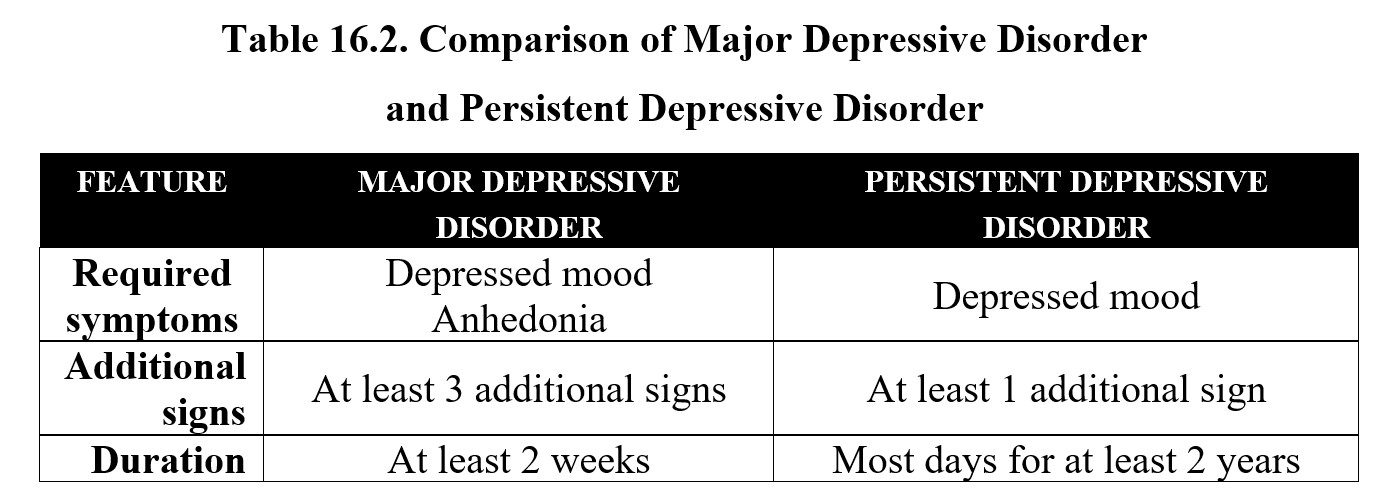
The other major classification of depression is persistent depressive disorder, which is also sometimes referred to as dysthymia. The only critical symptom here is a depressed mood, but instead of two weeks, the symptoms must occur on most days for at least two years. Persistent depressive disorder may also be accompanied by some of the signs mentioned above. Compare the diagnostic criteria for the two below:
Depression is one of the most common mental illnesses in the U.S., with around 8.4% of the population experiencing at least one episode of major depression in 2020. Depression is more common among women than men, with women being nearly twice as likely to develop depression (Albert, 2015). The exact symptoms shown vary and are influenced by gender, age, and the presence of other mental illnesses such as anxiety.
In addition, while we will not cover them for this class, there are other types of depression that you may see mentioned in the media or scientific literature. An example is a postpartum depression or “baby blues” that can occur after childbirth. Another common example is seasonal affective disorder (SAD), where depression is related to the decrease in daylight due to changes in seasons. These forms of depression share similar symptoms to the two main types described above but have unique causes.
Types of Depression and Bipolar Disorder in the DSM5 [8:06]
16.1.2. Monoamine Hypothesis of Depression
What are the causes of depression? There are many different possible answers to this question. For instance, many major depressive episodes show a clear precipitating event, such as the death of a loved one. Not all cases are like this, however, and some people are at a higher risk than others for developing depression. In the search for the underlying causes of depression, it was discovered that monoamine levels may form a biological basis for depression.
In particular, it was noted that low levels of norepinephrine and/or serotonin in the limbic system corresponded to symptoms of depression. The monoamine hypothesis of depression states that depression is caused by low levels of these monoamines.
What is the scientific method, and what is a hypothesis?
The scientific method is a cyclical process of problem-solving whereby facts are established. It begins with the formulation of a hypothesis based on preliminary observations. A hypothesis is a proposed explanation of the problem. The second step is to test the hypothesis and confirm or deny its validity. If the hypothesis is confirmed, it may be revised to be more specific. If the hypothesis is not confirmed, a new hypothesis may be formulated. The scientific method is a series of steps that are repeated over and over again, and the hypothesis is improved or made more specific each cycle (think of it as practice makes perfect). A hypothesis differs from a theory in that a hypothesis is a provisional or preliminary explanation that is undergoing testing for verification, whereas a theory is a tested and well-substantiated explanation that unifies facts, tested hypotheses, and evidence.
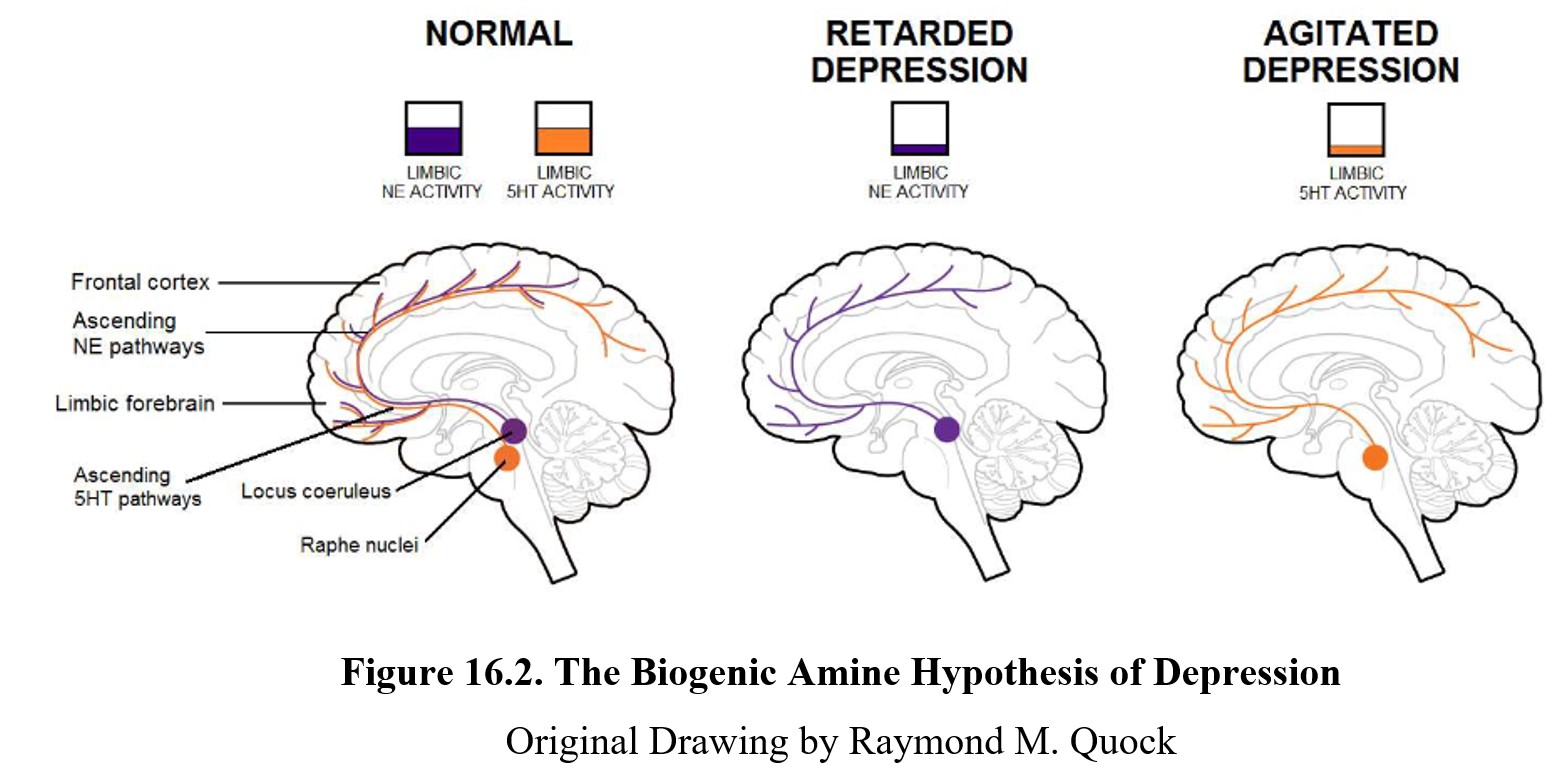
The brain on the left shows a healthy brain where levels of norepinephrine (NE) and serotonin (5-HT) are normal. These transmitters are involved in ascending pathways that start in the midbrain and project to different areas of the forebrain and cortex. These pathways and structures are involved in mood regulation in the brain.
Low levels of norepinephrine activity in these regions can lead to slowed thinking and actions (psychomotor retardation), symptoms commonly seen in those with major depression. Depression involving these symptoms is sometimes referred to as retarded depression. The “retarded” refers to the psychomotor retardation mentioned previously.
At the same time, low levels of serotonin activity corresponded to anxiety, irritability, racing thoughts, and restlessness. This type of depression is sometimes referred to as agitated depression. Because there is substantial overlap between various types of depression, it is sometimes diagnosed as depression with mixed features instead. Over ⅔ of major depressive disorders also meet the criteria for anxiety disorder (agitated depression).
16.1.3. Pharmacological Treatment
Pharmacological treatment of depression largely follows the monoamine hypothesis of depression. In other words, the goal of pharmacological treatment is to increase the amount of norepinephrine and/or serotonin transmission in the limbic system. The antidepressant drugs that we will discuss in the next section accomplish this through various methods, but the goal is always to increase the level of monoamine transmitters.
A potential challenge to the monoamine hypothesis is the fact that clinical improvement in mood is much slower than the increase in transmitter levels. When taking an antidepressant medication, the amount of neurotransmitter in the synapses increases within days. Despite this, the onset of improved mood can be slow and take up to 2–4 weeks. What might explain this latency?
One possible explanation is that the neurotransmitter deficiency is associated with an increase in receptor sensitivity. The real cause of improved mood after the medication is the normalization of receptor sensitivity. Although the amount of synaptic transmitter increases rapidly, it takes some time for the body to adjust to the new level and downregulate the receptors in response. This decrease in receptor sensitivity correlates more closely with clinical improvement in mood, as indicated in the chart below.
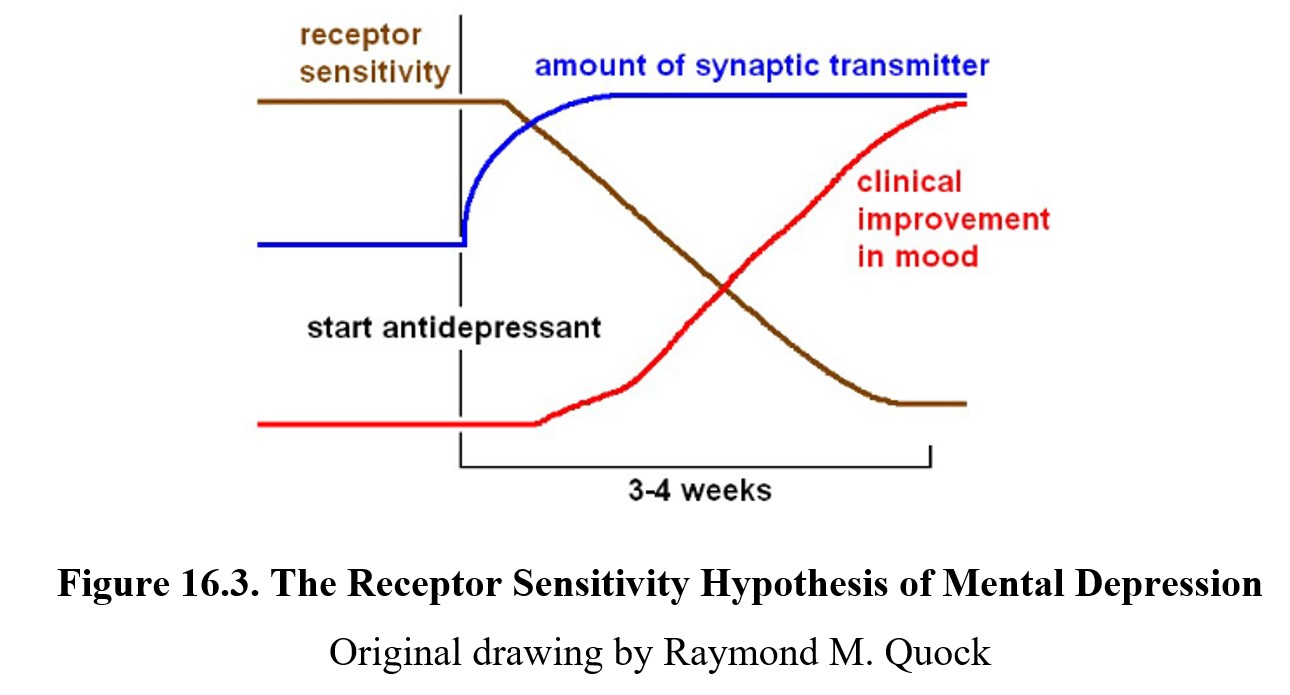
As time passed, further hypotheses emerged as possible explanations for the prolonged latency of antidepressant drug effects. These new ideas involve neuroadaptation in receptor signaling, changes in gene transcription, or changes in neurotrophic factors such as nerve growth factor (NGF), and brain-derived neurotrophic factor (BDNF).
A newer hypothesis is based on hippocampal neurogenesis. Recall that the hippocampus is a structure in the limbic system associated with learning and memory. Neurogenesis is the formation of new neurons in the brain. Although neurogenesis largely ceases once humans reach adulthood, the hippocampus is one of the few regions where neurogenesis continues into adulthood.
The hippocampus is particularly susceptible to stress. Although mild to moderate levels of stress can be tolerated, strong or chronic stress can disrupt the functioning of the hippocampus and lead to reduced attention, perception, and perception. In severe cases, such as the levels of stress seen in depression, this may lead to reduced neurogenesis and neurotoxicity.
Hippocampal neurogenesis is relevant to depression because studies have shown that antidepressants increase neurogenesis in the hippocampus. The time it takes for neurogenesis to increase is 2–4 weeks, similar to the time that it takes for clinical relief of depressed mood. As such, increased hippocampal neurogenesis may be the cause for the improved mood in people taking antidepressants.
16.1.4. Non-Pharmacological Treatments
There is a variety of non-pharmacological treatments for depression. A comm choice is psychotherapy which can be used on its own or in conjunction with antidepressant medications. Psychotherapy can help patients understand the causes of depression, feel more in control of their lives, and teach patients coping skills.
There are various approaches to psychotherapy for the treatment of depression. Individual therapy between the patient and therapist is most common. But other options include group therapy, couples therapy, and family therapy. Therapy may involve others to assure the patient that they are not alone in their experiences or to help the patient build a support network.
Another form of treatment is electroconvulsive therapy or ECT. In ECT, an electrical current is passed between two electrodes on each side of the brain to induce a brief seizure. The patient is under anesthesia during the treatment and side effects are generally limited to mild memory loss. Although the modern procedure is humane and consent is required, ECT is typically reserved as a last resort because of its negative portrayal in novels and films such as One Flew Over the Cuckoo’s Nest.
Scientists are not entirely certain why ECT is effective at treating severe depression, although it is hypothesized that the induced seizure acts to reset the brain somehow similar to rebooting a computer. Treatment involves giving ETC 2–3 times per week until a clinical improvement in mood is noted, which may take anywhere between 3–15 sessions. ECT has a higher success rate than any other form of antidepressant treatment besides esketamine (which we will discuss later) and can produce dramatic and life-saving results.
16.2. Antidepressant Drugs
Section Learning Objectives
- Differentiate between first- and second-generation (atypical) antidepressant drugs.
- Describe the main side effects of antidepressants and define antidepressant discontinuation syndrome.
- Discuss the mechanism of action of monoamine oxidase inhibitor (MAOI) antidepressants.
- Discuss potential drug interactions involving MAOIs.
- Discuss the mechanism of action of tricyclic antidepressants.
- Discuss the mechanism of action and drug interactions of selective serotonin reuptake inhibitor (SSRI) antidepressants.
- Briefly discuss other atypical depressants and the significance of esketamine.
Now that we understand the basis for pharmacological treatment of depression, it is time to examine specific types of antidepressant drugs.
Treating Depression with Antidepressants [11:09]
16.2.1. Overview of Antidepressants
Antidepressants can largely be split into two groups. First-generation antidepressants include monoamine oxidase inhibitors and some tricyclic antidepressants. These drugs were discovered earlier and tend to have greater side effects. By comparison, second-generation antidepressants were discovered more recently and have fewer side effects because they are more selective than first-generation antidepressants. Drugs in this group are also referred to as atypical antidepressants because they do not fit into the typical categories of first-generation antidepressants.
Although there are many types of antidepressants, almost all of them share the same general effects and side effects. Perhaps the most noteworthy effect of antidepressants is that they only elevate mood if it is depressed. Unlike stimulants such as amphetamine which produce stimulation followed by a compensatory depression, antidepressants do not cause rebound depression when stopped. Because of this, antidepressants are not habit-forming and are typically not misused (although there are exceptions—more on that later).
Are antidepressants stimulants?
Because we have previously framed CNS stimulants and depressants as opposing classes of drugs, it is reasonable to assume that anti-depressants are CNS stimulants. Unfortunately, this is wrong—in fact, antidepressants themselves are CNS depressants and can produce effects such as sedation.
The confusion here is that there are two uses of the word depression here. When referring to CNS depression or drugs like barbiturates, depression refers to an inhibition of CNS activity. In comparison, the use of depression in the term antidepressant refers to depressed mood. Notably, depressed mood and inhibition of CNS activity are not the same thing, which is why some antidepressant drugs can also inhibit CNS activity.
Common side effects of antidepressants include sedation, anticholinergic effects, orthostatic hypotension, sexual dysfunction, and weight gain. The cause of sexual dysfunction is uncertain but may be related to disturbance of acetylcholine or serotonin neurotransmission. Sedation and weight gain are attributed to the blockade of histamine H1 receptors. Anticholinergic refers to blocking muscarinic cholinergic receptors, which means a reduction in parasympathetic tone manifested as constipation, urinary retention, dry mouth, and increased heart rate. Orthostatic hypotension is caused by blockade of vascular alpha receptors and describes a sudden drop in blood pressure upon standing up.
Antidepressants vary in terms of which side effects are expressed and to what degree. This is important because side effects—in particular, sexual dysfunction and weight gain—can be highly undesirable to patients and cause them to stop taking their medication resulting in remission of their depression. The decision of which type of antidepressant to use is often determined by the side effects of the drug and any particular medical issues experienced by the patient. For example, a person with urination problems due to benign prostatic hyperplasia should not be given an antidepressant that is highly anticholinergic. A person with low blood pressure would not be given medication with a high incidence of orthostatic hypotension.
Finally, although antidepressants do not cause dependence, stopping antidepressant use can cause antidepressant discontinuation syndrome. This occurs in about 20–50% of patients following discontinuation after at least one month of use, with increased risks for longer treatment durations or medications with a shorter half-life. Symptoms include dizziness, nausea, headaches, irritability, and insomnia. It can be mitigated by gradually reducing the dose at the end of treatment, a process known as tapering, rather than abruptly stopping the drug.
16.2.2. Monoamine Oxidase Inhibitors
We will start with the earliest antidepressants discovered, the monoamine oxidase inhibitors (MAOIs). We have previously mentioned monoamine oxidase (MAO). There are two types of MAO referred to as MAO-A and MAO-B which differ in anatomical location and some substrate selectivity. MAO is bound to the other membrane of mitochondria and breaks down monoamine transmitters (norepinephrine, serotonin, and dopamine) within the presynaptic cell. MAO in the blood and liver can also help to break down monoamines such as tyramine found in foods.
The main physiological role of MAO enzyme is to regulate the amount of monoamine stored and available for release from presynaptic nerve terminals. When there is an excess of transmitter inside the nerve terminal, MAO goes to work to reduce them to normal levels. For example, following neuronal reuptake of transmitters from the synapse back into the neuron, monoamine transmitters may be degraded by MAO or repackaged into storage vesicles. These recycled transmitters will eventually be released into the synaptic space again.
Because both norepinephrine and serotonin are monoamine transmitters, they are typically metabolized or oxidatively deaminated by MAO enzyme. Consequently, as shown in the figure below, MAOIs can increase neuronal levels of both monoamines and increase norepinephrine and serotonin neurotransmission. Since there are deficiencies in norepinephrine and serotonin associated with retarded and agitated depressed states, respectively, MAOIs can produce antidepressant effects in both types of depression.
Although MAOIs were the first antidepressants discovered, nowadays they are rarely prescribed and are typically reserved for patients who are nonresponsive to other types of antidepressants. The main reason for the decline in use is that MAOIs are hepatotoxic and can also cause serious and potentially lethal drug or dietary interactions. MAOIs inhibit enzymes in the blood that are responsible for metabolizing other medications. This can cause dangerous interactions if MAOIs are taken alongside other drugs. In particular, the actions of indirect-acting sympathomimetic drugs such as pseudoephedrine (Sudafed®) may be potentiated.
MAOI treatment also requires dietary restrictions because of potential interaction with the substance tyramine. Tyramine is an amine naturally found in foods such as cheese, beer, beef, pepperoni, dried fruits, and many others. Normally, tyramine is metabolized by MAO, but when the enzyme is inhibited, tyramine levels can increase quickly. At high levels, tyramine acts as a weak amphetamine, inducing the release of large amounts of norepinephrine. This can lead to an acute hypertensive crisis or a severe increase in blood pressure that can be life-threatening.
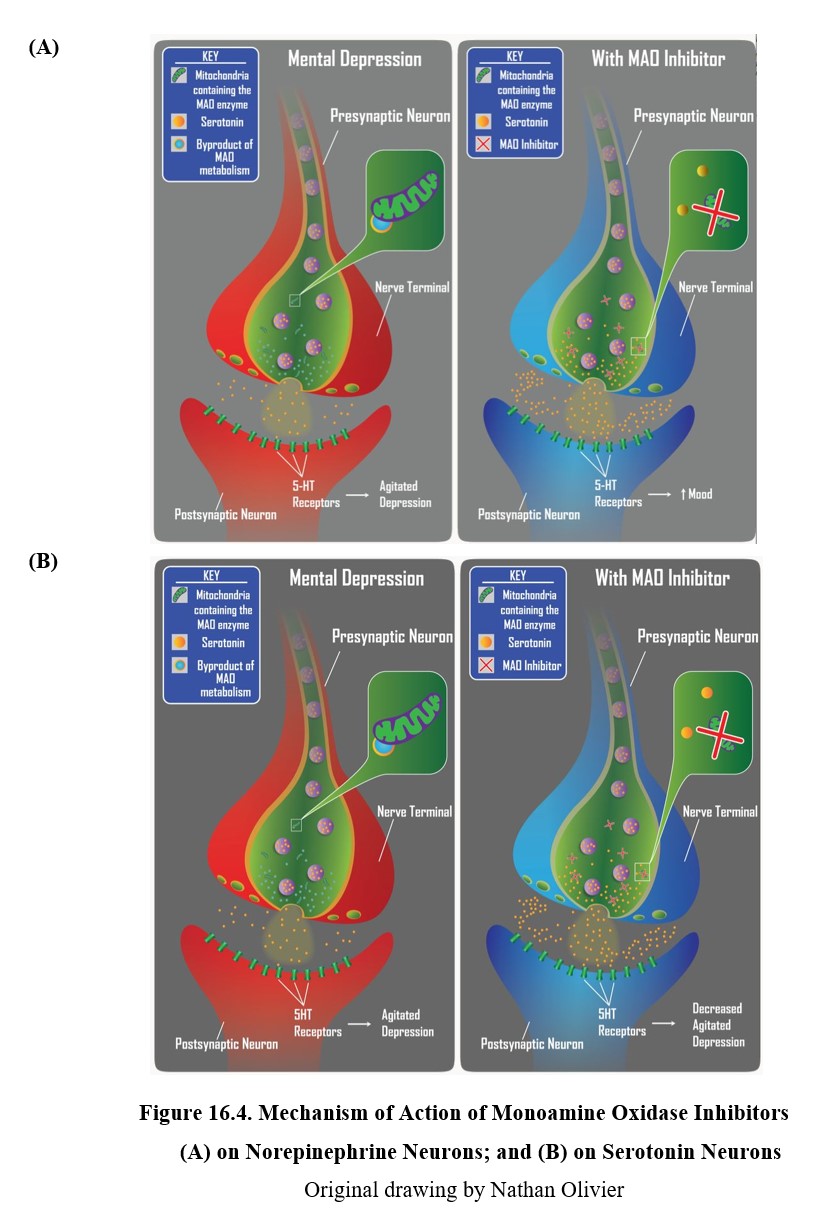
16.2.3. Tricyclic Antidepressants
Tricyclic antidepressants are part of the first-generation antidepressants along with MAOIs and were discovered around the same time. They are often abbreviated as TCAs or simply referred to as tricyclics. The tricyclic in the name refers to their chemical structure, which you can see in the two examples below. TCAs can be divided into two subgroups: secondary (2°) amine TCAs and tertiary (3°) amine TCAs based on how many carbon atoms are bound to the nitrogen. A secondary amine has two carbons attached to the nitrogen, and a tertiary amine has three. This will have consequences on the pharmacological properties of the drug.
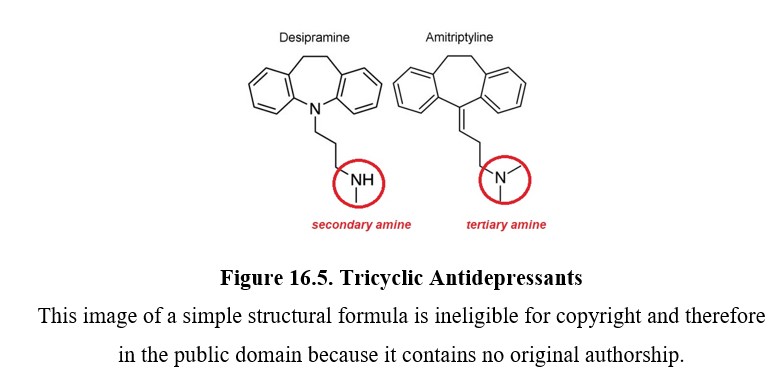
Tricyclic antidepressants inhibit the norepinephrine transporter (NET) and the serotonin transporter (SERT), which are responsible for neuronal reuptake of norepinephrine and serotonin, respectively. Secondary amine TCAs inhibit NET more than they do SERT, and tertiary amine TCAs block SERT more than they do NET. This means a difference in the transmitter that builds up in the synapse after treatment with each type of TCA.
TCAs do not have the same food and drug interactions as MAOIs, which made them a safer alternative to MAOIs. Compared to second-generation antidepressants, however, TCAs tend to have greater side effects. As you may recall, some of these side effects (especially sexual dysfunction and weight gain) can cause non-compliance, which leads to newer antidepressants being prescribed more often. Despite this, TCAs are still a good choice for depressed patients who are resistant to other medications.
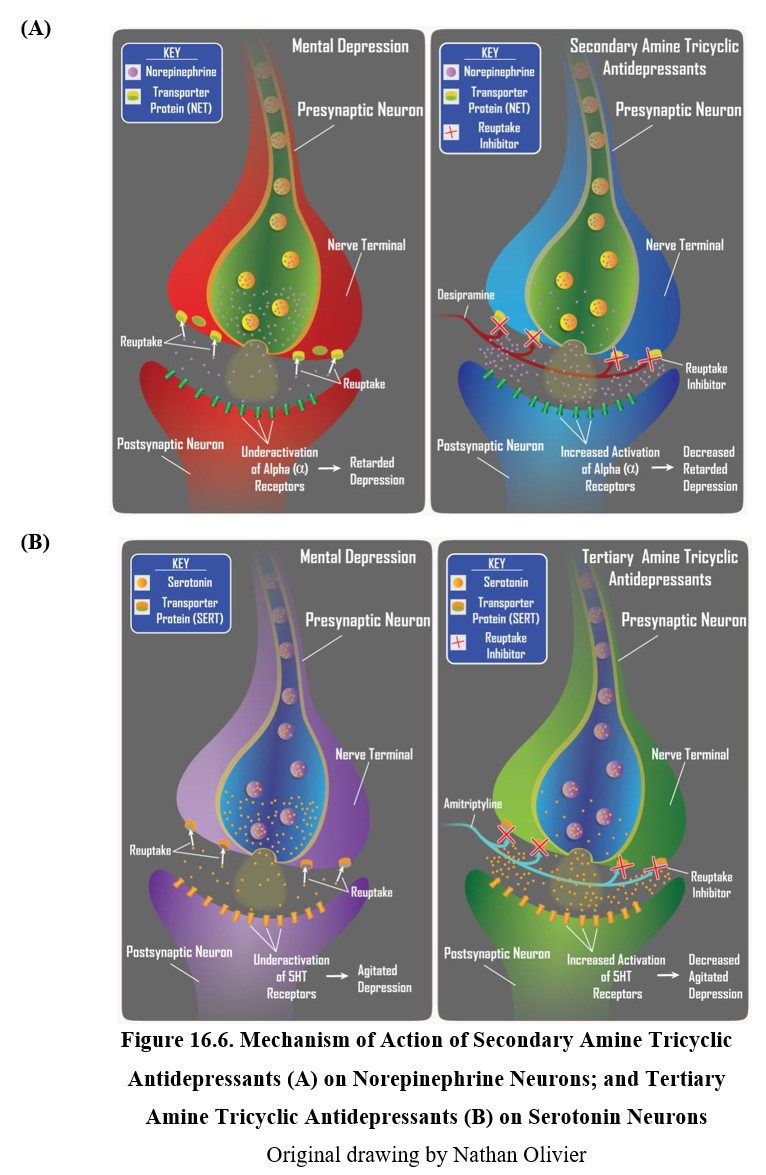
16.2.4. Selective Serotonin Reuptake Inhibitors
The first second-generation antidepressants discovered were selective serotonin reuptake inhibitors (SSRIs). As the name suggests, these drugs selectively inhibit serotonin reuptake, unlike TCAs (see figure below). Because they have a weak affinity for norepinephrine transporters, SSRIs are more useful for treating agitated depression than retarded depression but the distinction is not absolute.
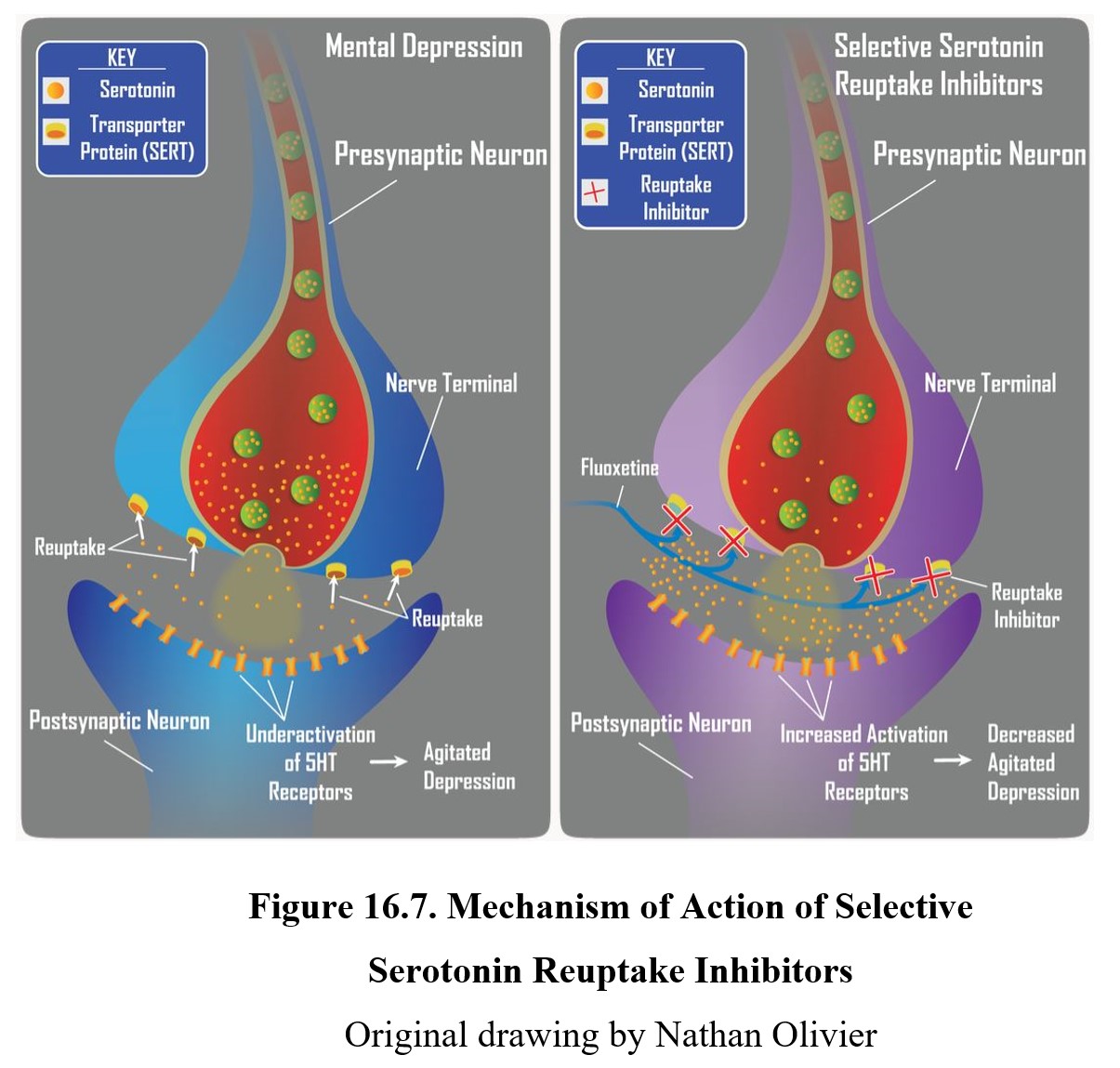
Perhaps the most significant SSRI is fluoxetine, marketed under the trade name Prozac®. Although it was not the first SSRI developed or sold, it had the largest impact and remains one of the most prescribed medications in the U.S. to this date (ClinCalc, 2021). Other commonly used SSRIs include sertraline (Zoloft®), citalopram (Celexa®), and escitalopram (Lexapro®).
When used as treatment for depression, SSRIs have similar effectiveness as TCAs (Anderson, 2000). The reason why SSRIs were considered a step-up was because they had fewer adverse side effects, making it more likely that patients would continue medication use. They also have a greater margin of safety because the toxic dose is higher than that of TCAs. The major side effects of SSRIs are sexual dysfunction and weight gain, which are the main reasons for n noncompliance and discontinuation of therapy.
SSRIs are not without their complications, however. In the 1990s, increasing reports of suicidal thoughts and behaviors under SSRI treatment led to FDA investigations and hearings on the matter. Over time, it was found that SSRIs and other antidepressant drugs could cause increased suicidality, especially in children and adolescents. This led to a public warning about the risk by the FDA in 2004, as well as the requirement to include a black box warning on antidepressant package inserts.
What might account for the increased suicidality after taking SSRIs? One explanation is the activating effect of SSRIs and other antidepressant drugs. This effect is an increase in energy, comparable to taking caffeine, that occurs rapidly after starting the drug treatment. Importantly, this effect occurs before clinical improvement in mood. Compare the onset of the two effects in the graph below.
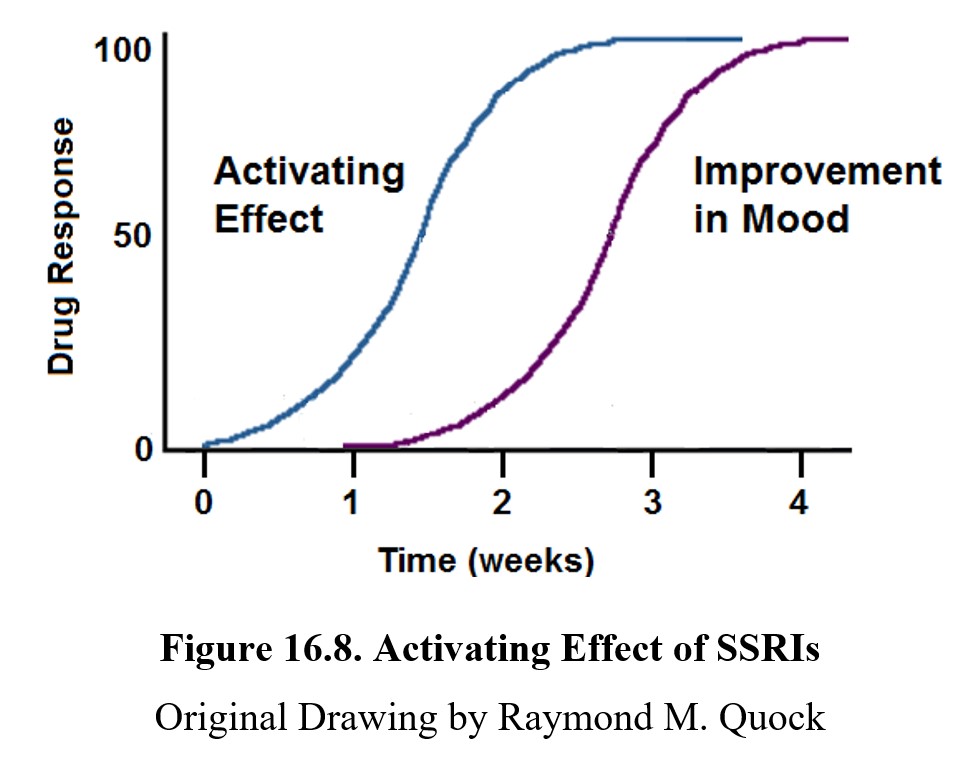
Because of this, during the first few weeks of treatment, patients may see an increase in the energy to act on suicidal impulses. Because the improvement in mood lags behind the activating effect, suicidality may increase for a brief period before decreasing. This effect is found in many SSRIs, especially fluoxetine (Prozac®).
Earlier, we mentioned how antidepressants are not habit-forming and are not usually misused, although there are some exceptions. Tapering or discontinuation of SSRIs may lead to antidepressant discontinuation syndrome. This is not the same as a withdrawal syndrome and should not be interpreted as physical dependence. A discontinuation syndrome is also associated with cardiac b blockers and the antihypertensive drug clonidine.
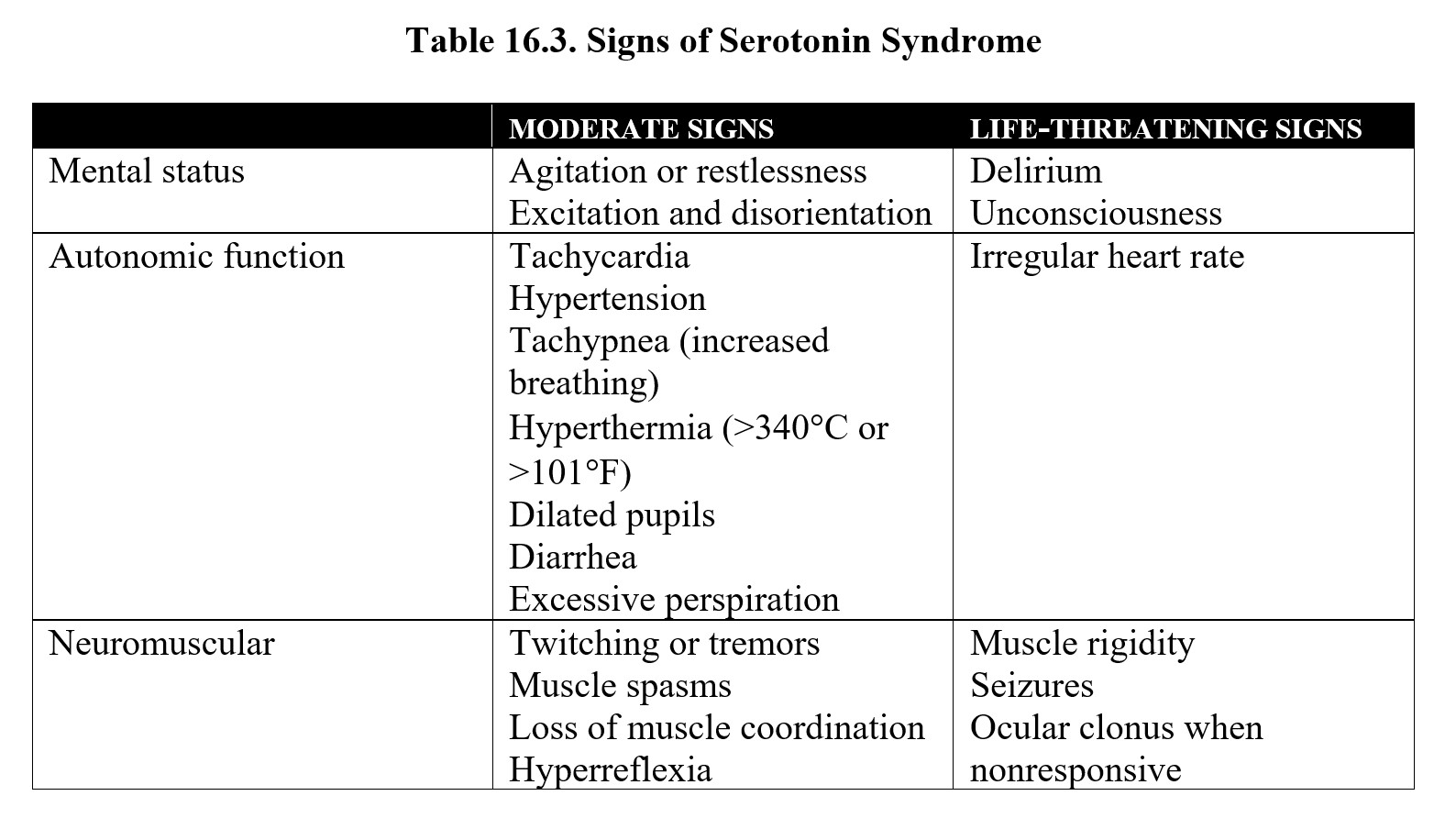
One final topic related to SSRIs that we should discuss is serotonin syndrome. This is a potentially life-threatening condition caused by an excessive accumulation of serotonin and the overactivation of serotonin receptors throughout the body. Serotonin syndrome can occur from any combination of drugs that increase serotonin levels, such as tricyclic antidepressants, selective serotonin reuptake inhibitors (SSRIs), serotonin/norepinephrine reuptake inhibitors (SNRIs), MAO-inhibitors, anti-migraine medications, and some opioid painkillers. Some herbal supplements such as St. John’s Wort and 5-hydroxytryptophan can increase serotonin levels and can also contribute to serotonin syndrome.
In mild cases, serotonin syndrome consists of increased blood pressure and heart rate, sweating, and pupil dilation (mydriasis). These symptoms intensify in moderate cases and are accompanied by agitation, disorientation, tremors, and increased reflexes. In severe cases, symptoms include hyperthermia, hypertension, delirium, and muscle rigidity. Antidotal therapy may involve benzodiazepines to relieve agitation or administration of a serotonin antagonist in extreme cases.
Serotonin Syndrome Made Simple [1:43]
SSRIs have also found their way into the drug scene at raves where it is misused to prolong the effects of other drugs. While SSRI themselves do not produce euphoria, they are notorious for interfering with the enzymatic activity of cytochrome P450. 3, 4-Methylenedioxymeth-amphetamine (MDMA, ecstasy) is inactivated by cytochrome P450 enzymes, so preadministraton or coadministration with SSRIs can prolong the psychoactive effects of ecstasy by 2-4 hours.
16.2.5. Other Atypical Antidepressants
Following the successful development of SSRIs, many other types of atypical antidepressants were created. Similar to SSRIs, these drugs were designed to selectively affect specific neurotransmitters. Most are reuptake inhibitors, but some are antagonists of presynaptic autoreceptors. Recall that autoreceptors are part of a negative feedback loop that inhibits further release of a neurotransmitter, so blocking autoreceptors increases transmitter release.
An up-and-coming antidepressant drug type is the serotonin/norepinephrine reuptake inhibitor (SNRI) which compares favorably with SSRIs. Representative SNRIs include venlafaxine (Effexor XR), desvenlafaxine (Pristiq®), and duloxetine (Cymbalta®). Today SNRIs and SSRIs account for the lion’s share of antidepressant drug prescriptions.
The table below contains a list of atypical antidepressants. It is not necessary to memorize all of them for this class. However, it is worth noting how the targets of antidepressants have changed. Newer antidepressants may be designed to interact with presynaptic receptors that regulate neurotransmitter release. Some drugs may inhibit reuptake of norepinephrine and serotonin such as tricyclic antidepressants but with greater specificity and fewer adverse effects.
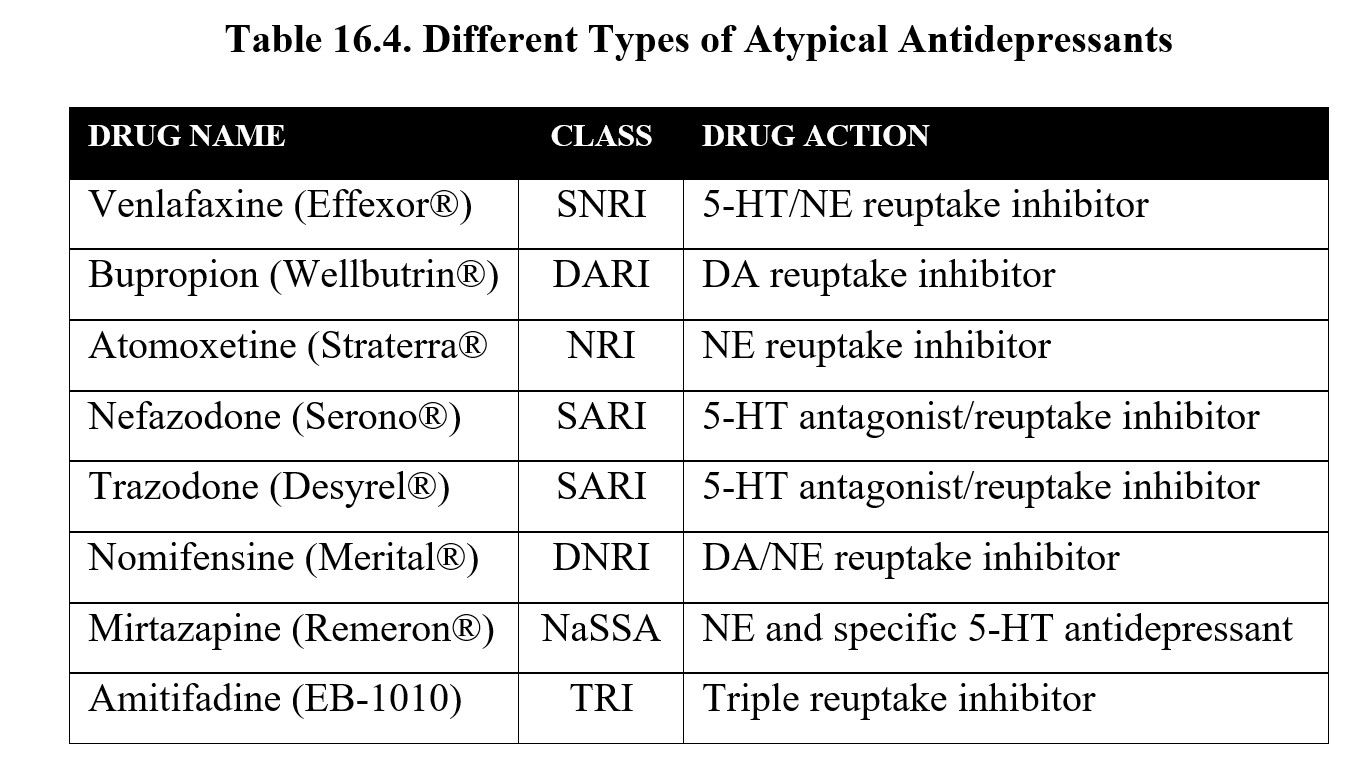
You may also recognize bupropion, which is also used to help people quit smoking and was mentioned in the chapter covering nicotine. Bupropion is the first drug to implicate dopamine in the regulation of mood.
Another significant discovery in antidepressant therapy was the discovery of the therapeutic potential of esketamine. As the name indicates, it is an enantiomer of the psychedelic drug ketamine, which is typically used as a racemic mixture. Unlike most antidepressants, a significant decrease in depressive symptoms can be seen as early as 2 hours after use. In 2019, it was approved by the FDA for use in treatment-resistant depression and is sold under the brand name Spravato® as a nasal spray.
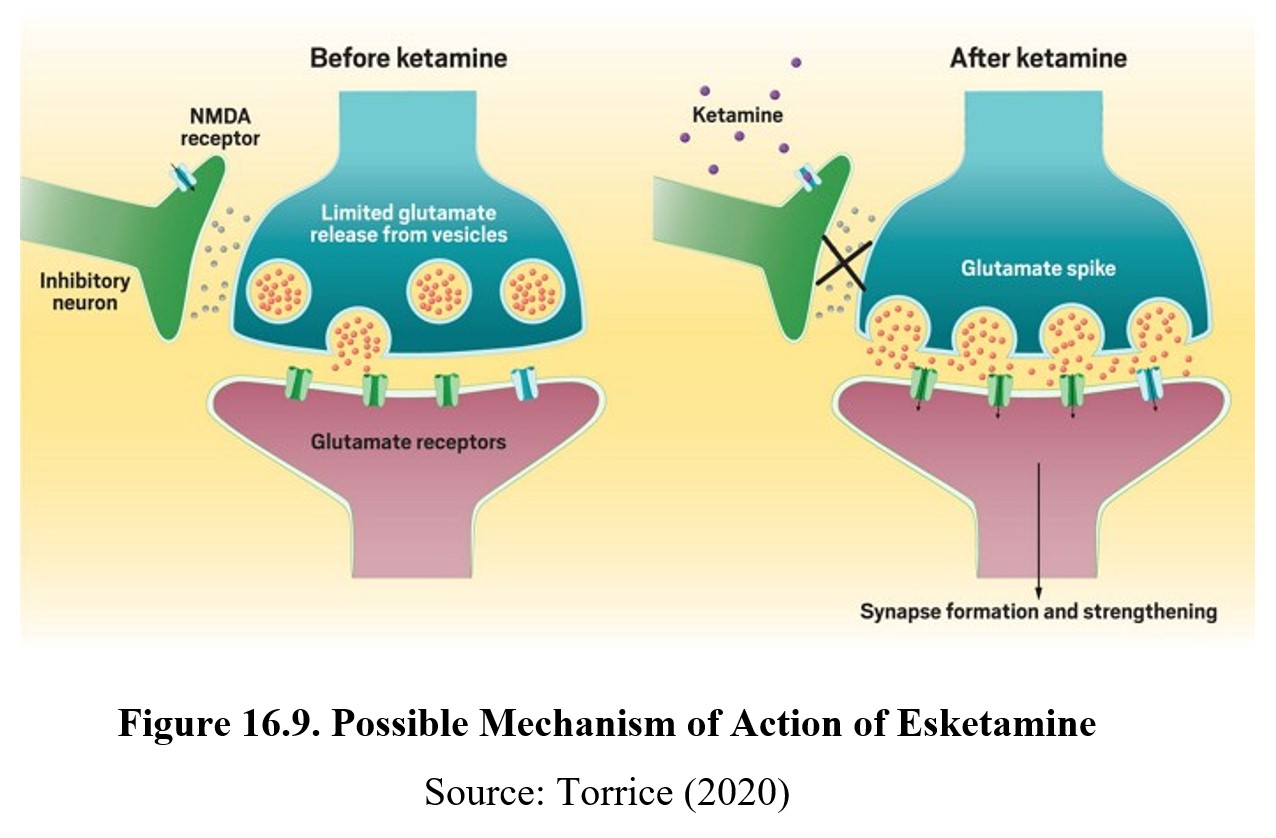
The presumed mechanism of action of esketamine is blockade of the NMDA receptor, which suggests a role for glutamate in the regulation of mood. An inhibitory neuron (GABA?) is hypothesized to prevent the primary neuron from releasing glutamate to elevate mood. Esketamine blocks NMDA receptors on the inhibitory neuron, thus lifting the inhibitory tone on the primary neuron. The increase in release of glutamate triggers synaptic formation and strengthening in the brain, possibly accounting for the rapid onset of action of esketamine.
16.3. Bipolar Disorder
Section Learning Objectives
- Describe the main signs of bipolar disorder.
- Differentiate between bipolar I disorder, bipolar II disorder, and cyclothymia.
- Describe the mechanism of action and effects of lithium carbonate and other mood stabilizers.
As mentioned near the start of this chapter, depression is not the only mood disorder. In this section, we will also cover bipolar disorder and the mood stabilizers used to treat it.
16.2.1. Symptoms of Bipolar Disorder
Bipolar disorder occurs when episodes of depression are accompanied by occurrences of mania or elevated mood. Because of this, the disorder used to be called manic depression. It is a severe condition that affects about 2.6% of the adult population. Due to the severity of the symptoms, almost 20% of bipolar sufferers die from suicide.
To gain an overview of the disorder, watch this video below. It covers much of the details that we will go over below in an interactive and easily comprehensible format:
What is Bipolar Disorder? [3:12]
To recap, bipolar disorder consists of both clinical depression and mania. We have already covered the signs of depression when discussing MDD in a previous section. Symptoms of a manic episode include high energy, delusions of grandeur, insomnia, loud and rapid speech, and high-risk behaviors. Mild cases of mania are referred to as hypomania.
There are three types of bipolar disorder. Bipolar I disorder is the most severe and only requires a single manic episode, although major depression is seen in the vast majority of cases. In comparison, bipolar II disorder requires a milder form of mania (hypomania) and major depressive episodes to be diagnosed. Finally, cyclothymia involves a history of both hypomania and brief episodes of mild depression.
16.2.2. Mood Stabilizers
Because there is no cure for bipolar disorder and manic episodes can be difficult to treat with traditional psychotherapy, management of the disorder usually involves long-term use of a mood stabilizer or medication that reduces extreme swings in mood. The first drug approved for the treatment of bipolar disorder, lithium, is the prototypical mood stabilizer and remains a popular choice in modern treatment.
Lithium is an elemental metal (meaning it can be found on the periodic table) and is usually prepared in salt form and stored in capsules when taken as medicine. It has a low therapeutic index, meaning its dosage must be very carefully controlled during treatment to avoid toxicity. A lithium blood test is used to determine whether lithium concentrations are within the therapeutic range. Side effects of lithium can include nausea, vomiting, and diarrhea. Lithium overdose may cause kidney failure, muscle rigidity, coma, and death.
Even at therapeutic doses, lithium can cause tremors, thirst, and increased urinary output. Commonly reported side effects include lethargy and weight gain. Because of this, noncompliance is relatively common in bipolar disorder compared to other mental illnesses. This can be exacerbated by the fact that multiple drugs are often used to treat bipolar disorder, which can cause unpleasant interactions and side effects. Drugs often used in conjunction with mood stabilizers include certain anticonvulsants and antipsychotics.
The exact mechanism of action for lithium and other mood stabilizers is unknown. However, various ideas have been suggested. One potential avenue is inhibition of the glycogen synthase kinase 3 (GSK-3) enzyme. Abnormal expression of this enzyme has been associated with increased risk for bipolar disorder, and lithium inhibits GSK-3 activity. This may, in turn, protect against neurodegeneration and aid in neuroplasticity.
Another possible explanation is the inositol depletion hypothesis. Inositol is a simple sugar involved in the synthesis of many different signaling compounds. One such compound is phosphatidylinositol, a signaling molecule that is found in excess in overactive neurons. Lithium has been shown to reduce cellular levels of inositol, which sequentially reduces phosphatidylinositol. Overall, this reduces the activity of overactive neurons and may exert an anti-bipolar effect. This pattern has also been seen in other mood stabilizers, such as valproic acid and carbamazepine.
Chapter Summary and Review
In this chapter, we covered the characteristics of two types of mood disorders, depression, and bipolar disorder, as well as the drugs commonly used to treat them. We began by defining depression and exploring how chemical imbalances may contribute to depressive symptoms. We discussed certain non-pharmacological treatments for depression, such as psychotherapy and ETC, before moving on to antidepressant drugs. In particular, we examined MAOIs, TCAs, SSRIs, and a handful of other atypical antidepressants, comparing their pharmacodynamics and side effects. We also distinguished between first- and second-generation antidepressants and defined conditions such as antidepressant discontinuation syndrome and serotonin syndrome. Finally, we defined the different types of bipolar disorder and explored the possible mechanisms of mood stabilizers such as lithium.
Chapter 16 Practice Questions
Answer the following questions:
- What is the difference between major depressive disorder (MDD) and persistent depressive disorder?
- What types of depression are low levels of norepinephrine and serotonin associated with, respectively?
- Provide two explanations for the latency of clinical mood improvement after starting antidepressant treatment.
- What is ECT? Describe the procedure and its effects.
- What are the five most common side effects shared among antidepressant drugs?
- Why did MAOIs fall out of favor?
- What is the difference between secondary and tertiary amine TCAs?
- Which of the following is an SSRI: desipramine, fluoxetine, esketamine, or tyramine?
- What is the activating effect of SSRIs and other antidepressants?
- Does serotonin syndrome cause low blood pressure?
- Modern atypical antidepressants accommodate for the potential role of a neurotransmitter besides norepinephrine and serotonin in depression. What is the transmitter?
- Differentiate between bipolar I disorder, bipolar II disorder, and cyclothymia.
- Does lithium have a high therapeutic index?
- What is GSK-3? How does lithium interact with it?
- How does the inositol depletion hypothesis connect inositol to bipolar behavior?
2nd edition