Chapter 10: CNS Depressants
2nd edition as of August 2022
Chapter Overview
Now that we have covered stimulants, it is time to move on to drugs that have opposing effects. In this chapter, we will examine a variety of depressants and learn about how they alter neurotransmission to reduce the activity of the central nervous system. We will begin with a review of the GABAA receptor which is the molecular target of a heterogeneous group of CNS depressant drugs ranging from alcohol to barbiturates to benzodiazepines and others.
Chapter Outline
10.1. CNS Depressants Overview
- 10.1.1. The GABA Receptor
- 10.1.2. Types of Depressants
- 10.2.1. Drug History and Overview
- 10.2.2. Administration and Pharmacokinetics
- 10.2.3. Mechanisms of Action and Effects
- 10.3.1. Drug History and Overview
- 10.3.2. Administration and Pharmacokinetics
- 10.3.3. Mechanisms of Action and Effects
- 10.4.1. Drug History and Overview
- 10.4.2. Administration and Pharmacokinetics
- 10.4.3. Mechanisms of Action and Effects
Chapter Learning Outcomes
- Identify and describe the receptor important for neural inhibition.
- Describe the history, administration, mechanisms of action, and effects of barbiturates.
- Describe the history, administration, mechanisms of action, and effects of GHB.
- Describe the history, administration, mechanisms of action, and effects of inhalants.
10.1. CNS Depressants Overview
Section Learning Objectives
- Describe the GABA receptor and discuss its importance in neural inhibition.
- Define sedative-hypnotics and provide examples of types of depressants.
As you would expect from the name, depressants are the opposite of stimulants. CNS depressants are drugs that reduce neuronal activity in the brain. Because of this they are sometimes colloquially referred to as “downers,” in contrast to the term “uppers” being used for stimulants. Although there are many different types of depressants, many target the same site of action: the GABA receptor. We have already acknowledged GABA as the most abundant inhibitory neurotransmitter in the CNS. Therefore, before we discuss any particular drugs, it is worth taking a closer look at this receptor and how different depressants interact with it.
10.1.1. The GABAA Receptor
Recall from Chapter 4 that γ-aminobutyric acid (GABA) is the brain’s main inhibitory neurotransmitter. This is because GABA targets GABA receptors, which promote hyperpolarization of the postsynaptic cell. This inhibits the postsynaptic cell from firing and releasing other neurotransmitters such as glutamate or norepinephrine. As a result, increasing GABA activity will, in general, reduce the activity of other neurons and transmitters.
There are two subtypes of GABA-sensitive receptors. The first type is the GABAA subtype. GABAA receptors are ionotropic receptors or ligand-gated ion channels. When they are activated, chloride ions (Cl-) flow into the cell, increasing the negative charge inside the neuron. By comparison, GABAB receptors are inhibitory metabotropic or 7TM GPC receptors. The G proteins are linked to potassium channels. The efflux of potassium ions (K+) increases the negative charge inside the cell. Hence, there are two different mechanisms by which the two GABA receptor subtypes cause hyperpolarization and neuronal inhibition. To review this information, you may find it helpful to watch this short video:
2-Minute Neuroscience: GABA [1:59]
GABAA receptors are comprised of five protein subunits surrounding the central chloride ion pore. The most common type of GABAA receptor has two α subunits, two β subunits, and one γ subunit, as seen in the diagram below. The primary binding site, also known as the orthosteric site, is where GABA normally binds to the receptor. The classical GABAA receptor is part of what is called the GABAA chloride channel receptor complex.
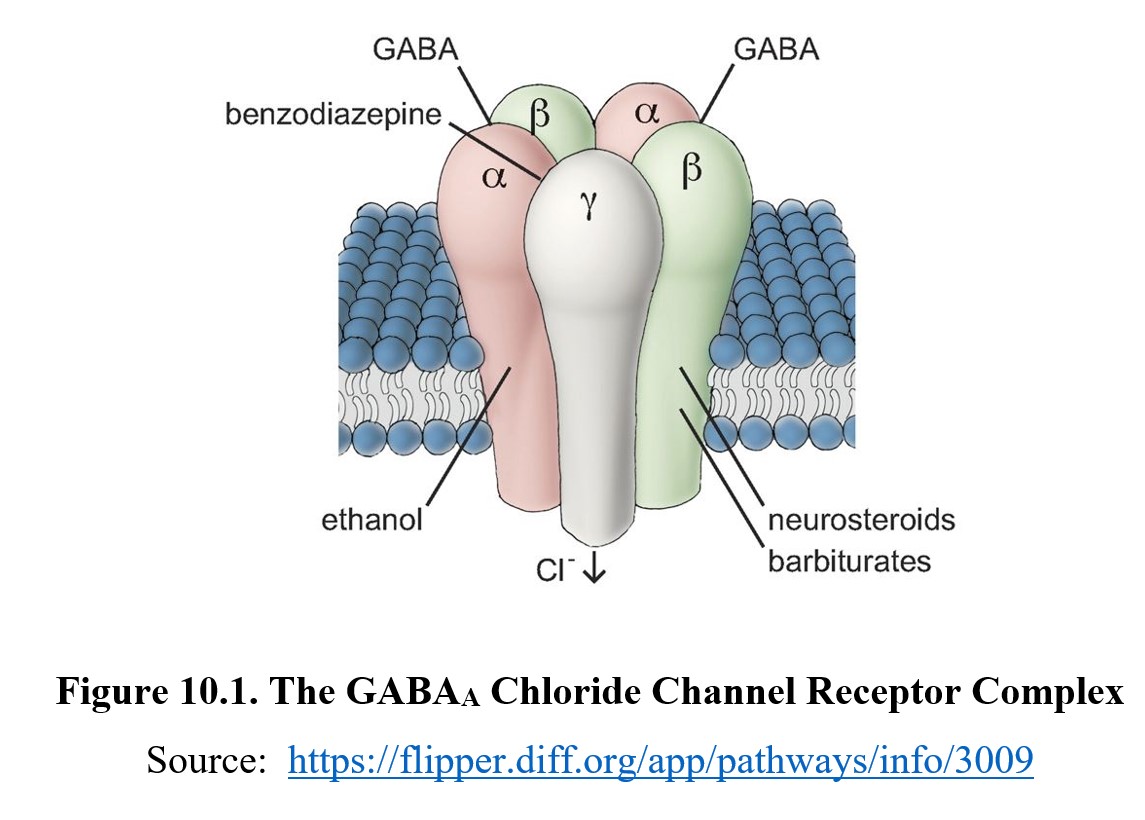
There are two orthostatic or primary binding sites on the chloride channel which interact with molecules of GABA. There are additional multiple allosteric sites that bind ligands other than GABA. This name should be familiar to you since we covered them in Chapter 4. To refresh, ligands that bind to these sites are called allosteric modulators. These change the functionality of the orthostatic site without competing for the same site.
Many depressants are allosteric modulators of the GABAA receptor. When they bind to the receptor, they change its conformation so that GABA has increased efficacy at the orthosteric site. Because they increase efficacy, they are known as positive allosteric modulators. Positive allosteric modulators do not increase the amount of GABA present in the synapse like reuptake inhibitors or activate the receptor on their own, as in the case of direct agonists. Instead, they change the conformation of the receptor so that it is more responsive to GABA binding. There are allosteric binding sites for various ligands, including benzodiazepines, barbiturates, and neurosteroids. As yet, an allosteric site where ethanol works is not known, although the inhibitory effects of ethanol are ultimately mediated through the GABAA receptor.
10.1.2. Types of Depressants
Many types of drugs produce depressant effects. Perhaps the most well-known depressant is alcohol. Because of its significance and certain unique properties, the entirety of the next chapter is devoted to covering it. Aside from alcohol, we will also find sedatives and hypnotics in this category. Sedatives calm anxiety and agitation, while hypnotics induce sleep. Since they share similar functions and many sedatives cause hypnotic effects at higher doses (and vice-versa), they are usually referred to as a single class of drug, sedative-hypnotics.
Sedative-hypnotics include barbiturates, benzodiazepines, and non-benzodiazepines (such as Z-drugs). We will discuss some of these in greater detail during Unit 4 on psychotherapeutic drugs, but, for this chapter, we will focus on barbiturates. Other types of drugs have sedative effects through action on the GABA receptor, such as γ-hydroxybutyrate (GHB), another drug we will be covering in this chapter.
Not all CNS depressants are sedative-hypnotics. Inhalants, which we will also be examining, do not have any sleep-inducing effects. At the same time, some drugs produce sedative effects through mechanisms other than the GABA receptor. Antihistamines, one such example, act at histamine receptors and cause drowsiness as a side effect. Although we will not be exploring them in this chapter, keep this in mind.
10.2. Barbiturates
Section Learning Objectives
- Explain the history and uses of barbiturates.
- Describe the pharmacokinetic properties of barbiturates and differentiate between barbiturates by duration.
- Define the pharmacodynamic properties of barbiturates and the consequences of barbiturate dependence and tolerance.
The first depressants we will discuss are barbiturates. Barbiturates are potent sedative-hypnotic drugs that were widely used in the early 1900s. Although their use has declined in recent decades, they remain an illustrative example of how depressants affect neurotransmission.
10.2.1. Drug History and Overview
Barbiturates are derived from barbituric acid, first synthesized in 1864 by the Bayer Company. No use was found for it until 1903 when German chemists discovered the sedative-hypnotic effects of its derived compounds. The first barbiturate, barbital, was marketed by Bayer under the name Veronal® that year, and barbiturate use steadily increased in the first half of the 20th century.
Barbiturates were routinely used to induce sleep in psychotic patients and were prescribed to treat insomnia and anxiety. They were also shown to reduce the number and intensity of seizures—a first since no other drugs were effective at treating epilepsy at the time—and began to see popular use as anticonvulsants. In 1912, Bayer produced another barbiturate, phenobarbital, which is still used to treat epilepsy to this day.
Dependence and overdose were identified as severe problems soon after the drug was synthesized. Despite this, barbiturates continued to be prescribed up until the 1950s and 1960s, when increased reports and greater visibility of barbiturate misuse led to significant change. Perhaps the most well-known instance of barbiturate overdose was Marilyn Monroe’s death in 1962. By 1970, barbiturates were considered controlled substances, and physicians were prescribing them at much lower rates.
Currently, most barbiturates are classified as Schedule III controlled substances, although some types, such as phenobarbital, are Schedule IV instead. Barbiturates have mostly been replaced with benzodiazepines and Z-drugs for treatment of insomnia and anxiety because they have fewer issues with dependence and overdose. They remain in use as anticonvulsants, general anesthetics, and antagonists to the effects of certain stimulants.
10.2.2. Administration and Pharmacokinetics
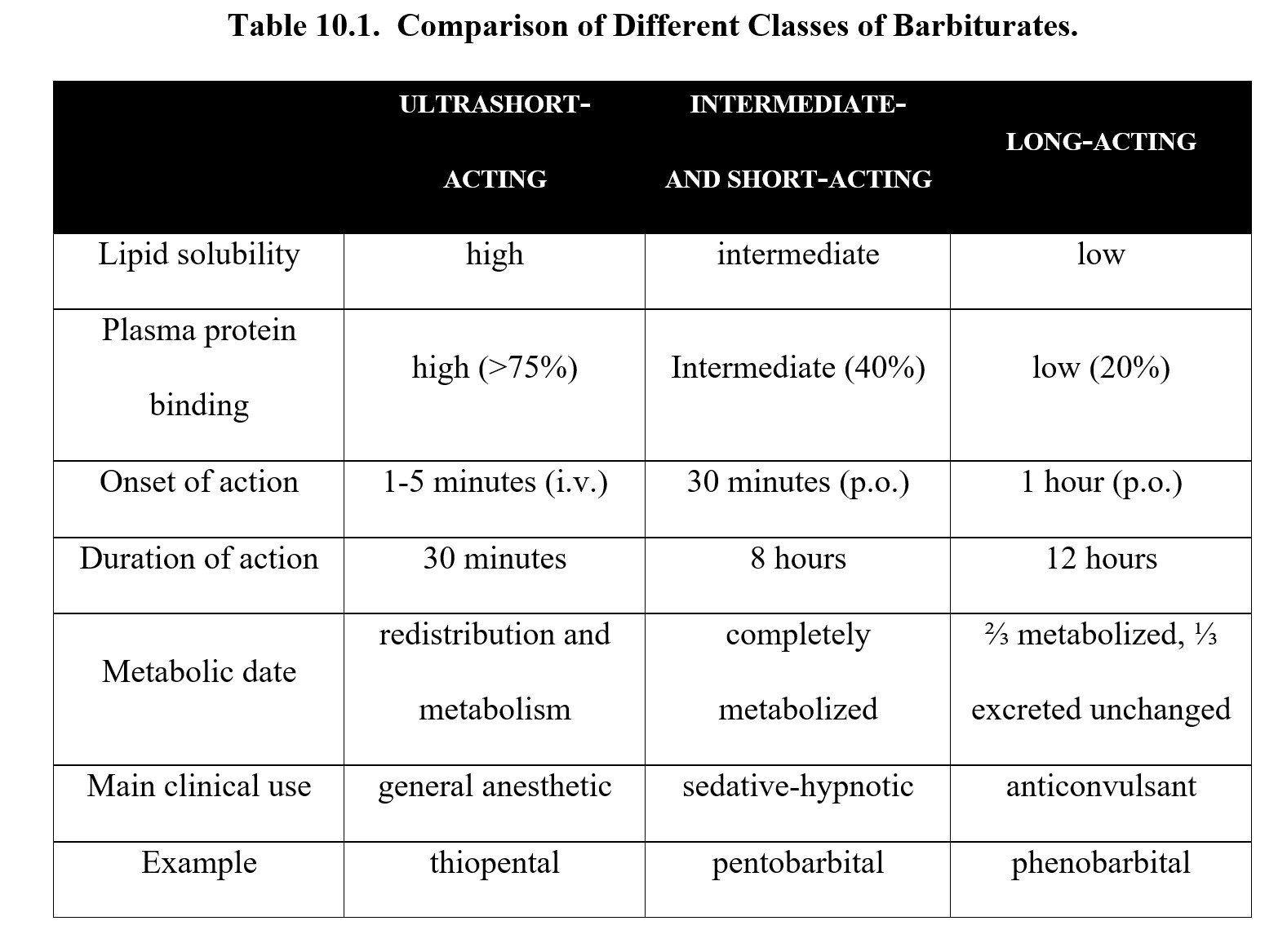
Barbiturates are generally classified by their duration of action. Long-acting barbiturates such as phenobarbital have low lipid solubility and are slowly absorbed. In exchange for a delayed onset (about 1 hour), effects can last for up to 12 hours. This makes them useful as anticonvulsants since fewer doses are required to maintain the level of drug in the body.
Intermediate- and short-acting barbiturates like pentobarbital and secobarbital have moderate lipid solubility. They are absorbed faster and have an onset of action of about 30 minutes, but their effects do not last as long as phenobarbital (up to 8 hours). The faster onset means these are used most often as sedative-hypnotics.
Ultrashort-acting barbiturates such as thiopental have the highest lipid solubility out of all barbiturates. Time to action can be mere minutes, although effects only last for around half an hour. Drugs like these are more suited for serving as general anesthetics for short surgical procedures.
Because they are weak acids, barbiturates are readily absorbed after oral administration. Other routes include rectal or intravenous. The method chosen depends on the intended use and recipient. Ultrashort-acting barbiturates are usually administered by IV, while long-acting anticonvulsant medications may also be taken by suppository.
Here is a table summarizing the information above:
10.2.3. Mechanisms of Action and Effects
Barbiturates are positive allosteric modulators of GABAA receptors. By binding to areas other than the orthosteric site of the receptor, they enhance GABA activity. In particular, they increase the amount of time that the chloride ion channel remains open when GABA binds to the receptor. At high concentrations, barbiturates can also bind to the main site as direct agonists.
At the same time, barbiturates are also antagonists to certain glutamate receptors. Recall that glutamate is an excitatory neurotransmitter. By blocking these glutamate receptors—NMDA, AMPA, and kainate—barbiturates further reduce CNS activity. This accounts for the strong effects of barbiturates compared to other sedative-hypnotics. Thus, barbiturates not only enhance inhibition but also block excitation.
The effects of barbiturates are dose-dependent. At lower doses, they produce sedation and hypnosis. But higher doses can induce deeper and deeper stages of depression—anesthesia, coma, and even death. These effects tend to follow one another in sequence as you increase the dose (see figure below):

Intermediate-acting barbiturates used as sedative-hypnotics can induce sleep. Specifically, they reduce the time needed to fall asleep, increase the time spent asleep, and reduce the occurrence of rapid eye movement (REM) sleep.
Stages of Sleep – non-REM, REM, Sleep Studies [3:40]
A negative effect of barbiturates on sleep is the ability of repeated drug treatment to interfere with rapid eye movement (REM) sleep. Sleep can be divided into two basic types: orthodox (non-REM) sleep and paradoxical (REM) sleep. It is the amount of REM sleep that is critical for restoring health in brain regions essential to learning and memory. During REM sleep, the brain is very active, wherein mixed frequency brain wave activity mimics the awake state. Dreaming occurs during REM sleep. Repeated use of barbiturates causes REM suppression which reduces the quality of sleep. If repeated use of barbiturates is stopped, there is REM rebound which reduces the quality of sleep by repeated nightmares.
At high doses, barbiturates can result in generalized CNS depression. Symptoms include loss of muscle coordination, difficulty thinking and speaking, and shallow breathing. These symptoms often result in behavior similar to that exhibited by someone who is drunk. Eventually, these symptoms can worsen and, uncorrected, lead to respiratory depression, coma, or death.
Being derivatives of barbituric acid, barbiturates are weak acids. As such, their reabsorption in the renal tubules can be affected by urinary pH. In the case of poisonings, an antidotal measure is acidification of the urine with ammonium chloride. The reduction in urinary pH causes more and more of the barbiturate to become ionized. This, in turn, impedes its ability to be reabsorbed from the renal tubules back into the circulation. Thus, the excessive barbiturate in the body is eliminated in the urine. This is exactly the opposite from how to treat an overdose with a weak base such as amphetamine.
As mentioned earlier, barbiturate dependence is noted to be a considerable problem. Tolerance to the sedative-hypnotic effects of barbiturates will develop with repeated use, but the same cannot be said for toxic effects such as respiratory depression. This means that over time, the therapeutic index for barbiturates grows smaller and smaller as the dose-response curve shifts to the right (see dose-response curve below), but there is little tolerance development to respiratory depression. Consequently, the barbiturate-tolerant individual keeps increasing the dose needed for euphoria until it catches up with the lethal dose.
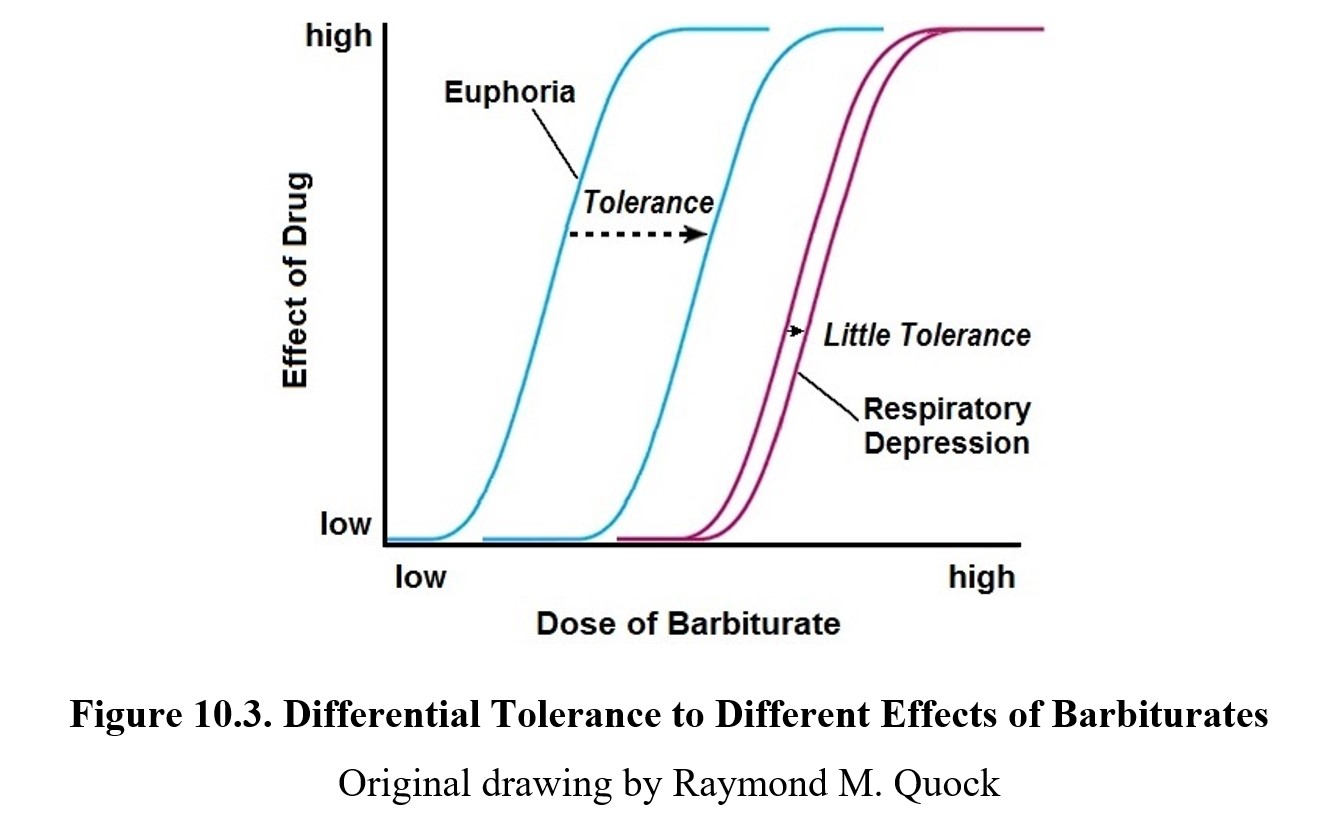
Withdrawal symptoms include anxiety, insomnia, nausea and vomiting, muscle weakness, abdominal cramps, and increased heart rate. At high levels of dependence, these symptoms are exacerbated, and withdrawal may involve convulsions, hallucinations, delirium, cardiovascular collapse, and death. Treatment for barbiturate dependence involves detoxification and gradual reduction in symptoms of dependence. Fortunately, the withdrawal symptoms can be suppressed by safer sedative-hypnotic drugs like benzodiazepines.
10.3. GHB
Section Learning Objectives
- Explain the history and uses of GHB.
- Describe the pharmacokinetic properties of GHB.
- Describe the pharmacodynamic properties of GHB and the role of GHB and GABAB receptors.
The next depressant we will examine is gamma-hydroxybutyric acid (GHB). It is an endogenous substance that can also be taken as a medication or used recreationally. Although it primarily acts as a depressant, it causes biphasic effects, with stimulatory effects occurring at low doses or for a short time initially. Because of this, it is primarily used as a club drug.
10.3.1. Drug History and Overview
GHB is both an endogenous neurochemical as well as an exogenous chemical compound. GHB was first studied in-depth in the 1960s for its potential use in treating narcolepsy and alcoholism. Although there was little support for its use in treating alcoholism, the salt form of GHB, sodium oxybate, is still used for the treatment of narcolepsy to this day under the brand name Xyrem®. GHB is an example of a drug that is listed in both Schedules I and III, depending upon the intent of use. A precursor to GHB, gamma-butyrolactone (GBL), has also been classified as a Schedule I controlled substance.
In the 1990s, GHB was marketed as a dietary supplement and found some use among athletes as a performance-enhancing drug, despite a lack of evidence for any performance-enhancing effects. It also gained a reputation among bodybuilders for increasing levels of growth hormone leading to increased muscle mass and reduced fat.
GHB found its main use as a club drug or party drug because of its euphoric effects at low doses. It is also easier to manufacture than most other club drugs, making it an attractive alternative. GHB is also occasionally used as a date-rape drug due to the drug’s ability to induce unconsciousness and amnesia. It is colorless and odorless and can be easily poured into a drink without notice. Although its use as a date-rape drug has been highly publicized, it is difficult to know how frequently it is used this way since there are several different drugs used for date rape such as flunitrazepam (Rohypnol®) and ketamine.
10.3.2. Administration and Pharmacokinetics
GHB is typically taken orally as a dissolved powder or a solution. It is absorbed quickly and reaches peak concentration in the blood at around 45 minutes. Effects can begin to show as early as 20 minutes after administration and last up to 2 hours.
GHB is metabolized rapidly and has a short half-life of about 30 minutes. Because of this, GHB is eliminated from the body faster than most drugs and can only be detected for 8-12 hours after its administration. This is part of the reason why the use of GHB as a date-rape drug is hard to track. A urine sample would need to be analyzed within a day of the suspected administration for there to be any chance of getting a positive result.
When GHB and alcohol are combined, the sedative and depressant effects are amplified, and GHB may reduce the rate at which alcohol is eliminated from the system. This synergistic interaction can lead to unexpected respiratory failure and death.
10.3.2. Mechanisms of Action and Effects
The following video is a succinct review of GHB, a brief history, illicit uses, mechanism of action, its dose-related pharmacological effects, illicit uses, and a clinical case scenario.
GHB is an endogenous neurotransmitter that is synthesized in GABA neurons. It is stored and released together with GABA from nerve terminals. There appears to be a dynamic relationship between GHB and GABA in that each is a precursor as well as a by-product of the other.
At physiological concentrations, GHB the neurotransmitter has affinity and efficacy for specific GHB receptors that are excitatory GPC receptors that evoke a stimulatory response. These receptors enhance glutamate activity and stimulate dopamine and serotonin release. This is where the stimulatory effects of GHB come from. The release of dopamine due to GHB receptor activity also contributes to the addictive properties of the drug.
At higher pharmacological concentrations, GHB the drug activates GABAB receptors, which is the mechanism of its CNS depressant properties. The differential actions of GHB on GABAB and GHB receptors likely explain the biphasic depressant and stimulatory effects of GHB with decreasing concentrations of GHB in the system.
Early effects of GHB consist of stimulation, relaxation, euphoria, and increased energy. Some users report hallucinations and aphrodisiac effects. As time goes on, users begin to exhibit symptoms similar to alcohol intoxication, including reduced inhibitions, impaired motor coordination, and slurred speech. At high doses, toxic effects such as nausea and vomiting, slowed heart rate, low blood pressure, convulsions, coma, and respiratory failure can occur. After use, people will experience fatigue, amnesia, confusion, and anxiety.
GHB is highly addictive and repeated use can lead to the rapid development of tolerance. This is somewhat undercut by the drug’s short half-life (t½ = 30-60 minutes). Users would have to take the drug very frequently, making addiction somewhat rare. Despite this, it is still possible to develop dependence from constant use. Withdrawal symptoms can be very dangerous and include tremors, seizures, and insomnia.
10.4. Inhalants
Section Learning Objectives
- Define inhalants and describe types of inhalants and the prevalence of inhalant use.
- Describe the pharmacological properties of inhalants.
- Differentiate between different methods of inhaling.
- Describe the four stages of inhalant-induced CNS depression and explain sudden sniffing death syndrome.
- Describe the use and misuse of nitrous oxide.
For the last section of our chapter on depressants, we will cover a type of drug that many people might overlook. Inhalants are solvents or other materials that produce vapors that elicit psychoactive effects. While a wide variety of products can be used as inhalants, most induce CNS depression through similar mechanisms of action.
10.4.1. Drug History and Overview
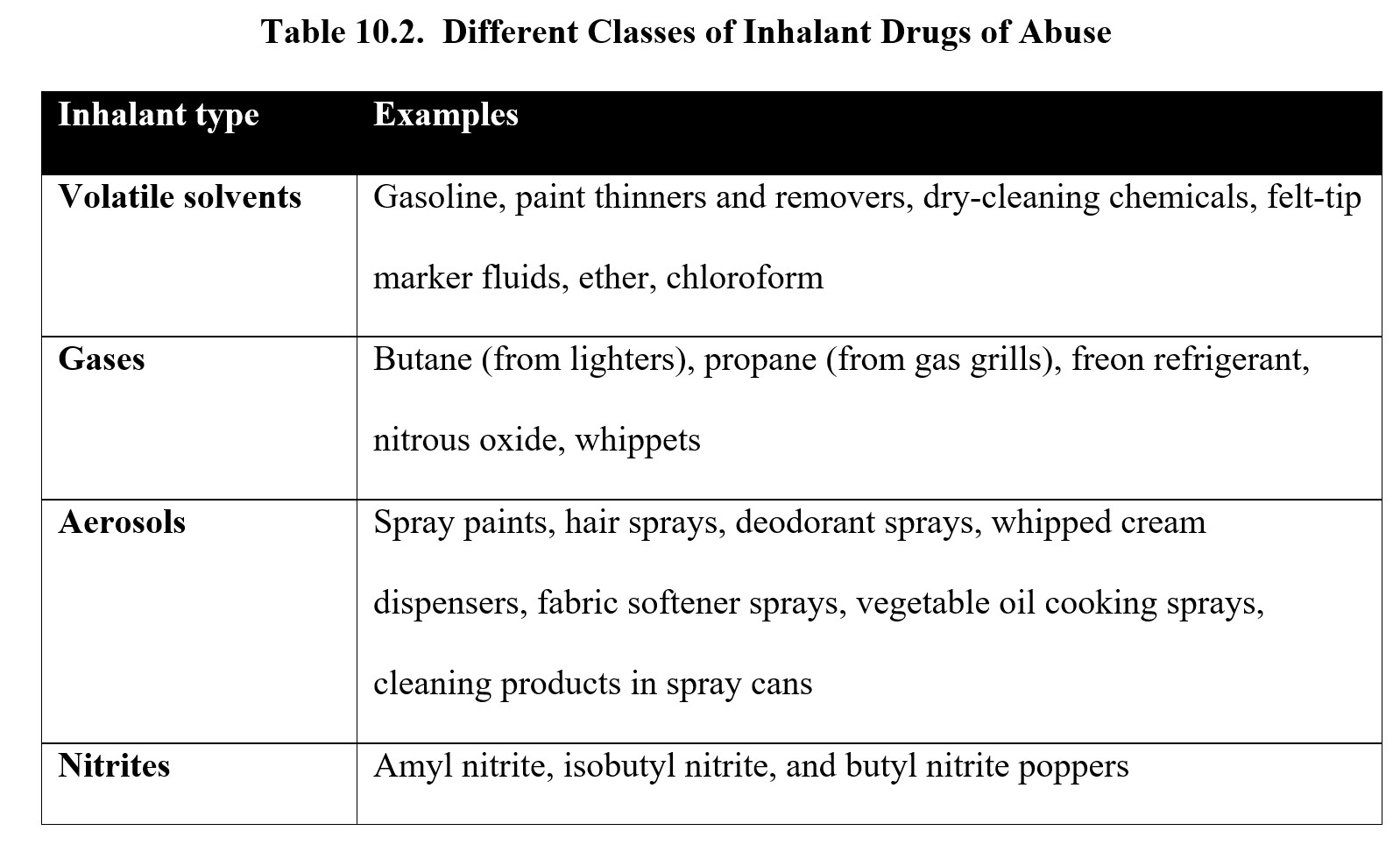
Inhalant is an umbrella term that refers to numerous chemicals that can be inhaled to produce intoxication. These chemicals can be found in various household goods and cleaning supplies such as glues, aerosol sprays, paint thinner, nail polish remover, gasoline, whipped cream, and felt-tip markers. A notable inhalant is nitrous oxide, a gas used as an anesthetic in surgeries and dentistry. These substances are typically unregulated and can be easily purchased or found in products around the house.
Unlike other psychoactive drugs, inhalants are most commonly used by children and adolescents. It is estimated that one in four grade school and middle school students have intentionally used a common household product to get high by the time they reach the eighth grade. For most, inhalants are the first abusable drugs encountered due to curiosity but rarely a deliberate attempt to get high. Studies have shown that as users age, they tend to use inhalants less often. Because of their widespread use by children, inhalants are reportedly the fourth-most misused substance after alcohol, tobacco, and marijuana.
10.4.2. Administration and Pharmacokinetics
As indicated by the name, inhalants are administered by inhaling the substance over some time at high concentrations. There are multiple ways the chemicals can be inhaled.
- Sniffing: inhaling the vapors from a soaked cloth or open container.
- Huffing: placing a soaked rag cloth directly over the mouth and nose to inhale. Bagging: spraying or pouring the liquid into a paper or plastic bag and placing it over the head.
- Spraying: spraying the aerosol directly into the nose or mouth.
- Inhaling: filling a balloon with nitrous oxide and inhaling from it.
Most inhalants are lipid-soluble and are absorbed very quickly, with concentrations in the blood peaking close to the time of administration. The combination of fast absorption and taking in the drug through the lungs results in an immediate rush and noticeable effects. Metabolism and excretion vary depending on the chemical in question, but half-lives tend to be very short. Nitrous oxide, for instance, is exhaled almost entirely through the lungs unchanged, resulting in a half-life of about 5 minutes. On the other hand, huffing produces an effect lasting 30-45 minutes.
10.4.3. Mechanisms of Action and Effects
Because inhalants are a heterogeneous collection of chemicals, it can be difficult to summarize drug actions. In general, however, there are two common mechanisms. Inhalants often are allosteric modulators of GABAA receptors as well as antagonists at glutamate NMDA receptors. Both actions result in decreased CNS activity and depressant effect.
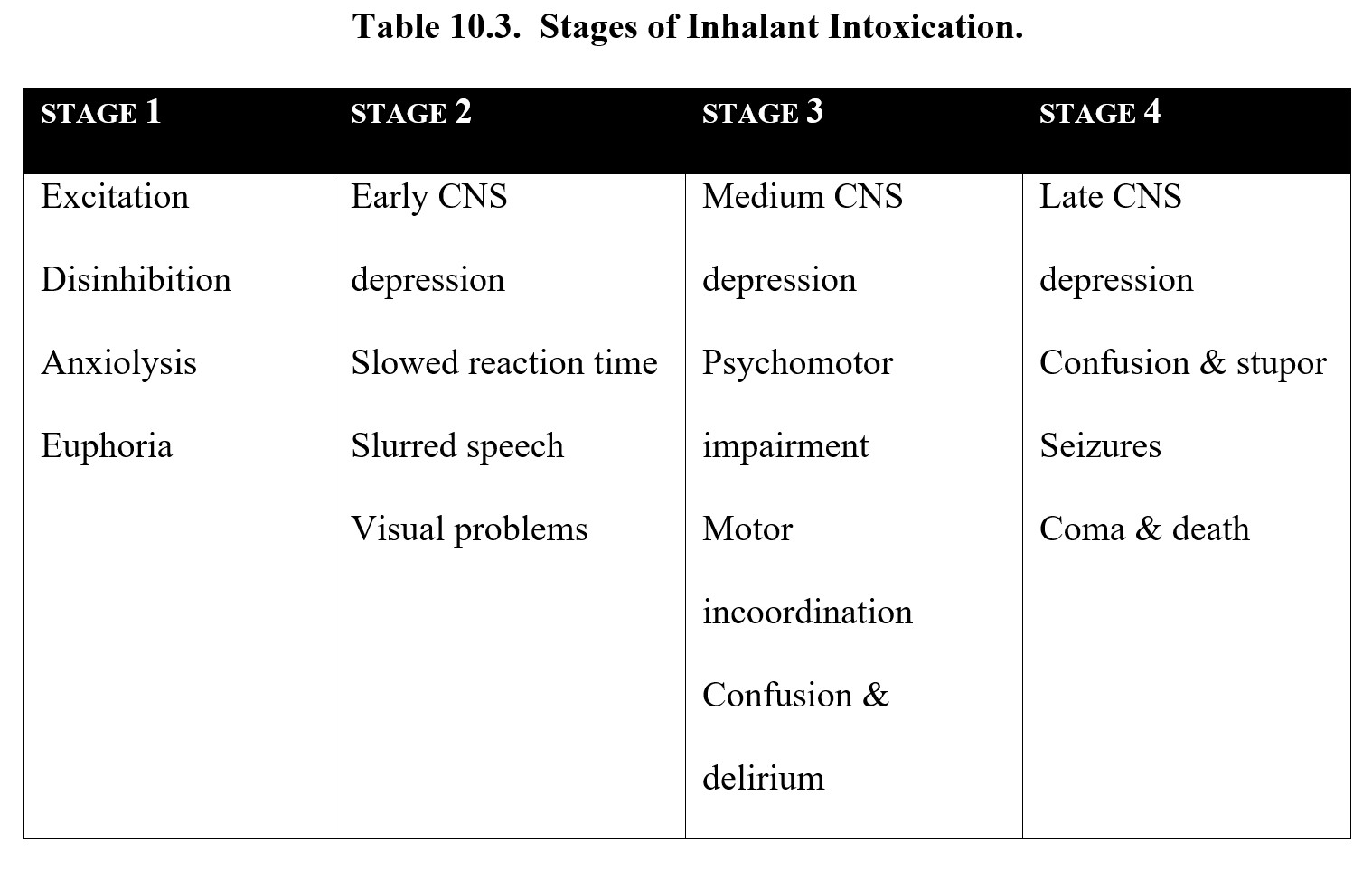
The exact effects of inhalants also vary, but they typically follow four stages (see figure below). Stage 1 is the excitatory stage, where the user experiences euphoria and agitation. This turns into Stage 2, early CNS depression, which is characterized by slurred speech and hallucinations. In Stage 3, medium CNS depression, the user experiences confusion, delirium, and impaired muscle coordination (ataxia). Finally, Stage 4 is late CNS depression, which can cause stupor, seizure, coma, and death.
Inhalants as Drugs of Abuse [3:20]
The vapors in inhalants compete with oxygen, which means users of inhalants may have a low oxygen supply. Coupled with slowed breathing or respiratory failure, this may result in a delayed death. However, inhalants are known to cause immediate arrhythmias and cardiac arrest, known as sudden sniffing death syndrome. This can occur after a single misuse and be fatal.
It is worth taking a closer look at nitrous oxide (N2O). As mentioned earlier, nitrous oxide is used as a general anesthetic. It also has analgesic (pain-relieving) and anxiolytic effects. Nitrous oxide is often misused because it is unregulated and produces euphoria and giddiness, which is why it is also called laughing gas. It can also lower inhibitions and cause dissociation, unconsciousness, dizziness, and loss of motor function. There is evidence that nitrous oxide is an NMDA receptor antagonist so its mechanism of action may differ from other inhalants.
When used in a medical or dental setting, a mixture of nitrous oxide and oxygen is dispensed by an anesthesia machine with a fail-safe system to protect the patient from hypoxia. The system shuts down the delivery of nitrous oxide if the oxygen content falls below 30% (the concentration of oxygen in room air is 21%). Another safeguard for nitrous oxide use is scavenging systems to remove nitrous oxide from the air and prevent toxicity in patients and dental staff.
Chronic exposure to nitrous oxide inactivates Vitamin B12, a cofactor in the methylation pathway for DNA and protein synthesis. Clinically, this can be manifested as megaloblastic anemia, a condition caused by improper production of erythrocytes (red blood cells).
A common mode of nitrous oxide recreational use is inhalation or collection of the gas from cooking sprays and whipped cream canisters that use nitrous oxide as the propellant. Frequent abuse of nitrous oxide in this manner not only produces a short-lasting but intense high but also results in hypotension, heart attack, and Vitamin B12 deficiency (Stockton et al., 2017).
Hippie Crack: The Dangerous Underworld of Nitrous Oxide Dealers [4:19]
Chapter Summary and Review
In this chapter, we learned about how depressants reduce CNS activity through GABA and examined a few different types of depressants. We started with barbiturates and learned about their sedative-hypnotic effects and risks associated with dependence. We then moved on to GHB and discussed its biphasic effects. Finally, we discussed the effects and misuse of inhalants such as nitrous oxide.
That will be all for this chapter. For the last chapter in this unit, we will take a detailed look at alcohol, the most infamous depressant of all. Much of the terminology used to describe alcohol’s effects will have already been introduced in this chapter, so make sure you are comfortable with this chapter’s material before moving on.
Chapter 10 Practice Questions
Answer the following questions:
- Both GABAA and GABAB receptor subtypes cause depolarization. What are the two different ways that they achieve this?
- What is a positive allosteric modulator? How does this compare to a direct agonist? (Bonus question: What do you think a negative allosteric modulator does?)
- Methohexital is a barbiturate with a rapid onset of action that is typically used for anesthesia. What type of barbiturate is it classified as?
- Why is barbiturate dependence likely to result in an eventual overdose?
- Are GHB receptors excitatory or inhibitory?
- Describe the biphasic effects of GHB. What are some symptoms from both phases?
- Classify the following drugs by their schedules: barbital, phenobarbital, GHB, and sodium oxybate.
- Does inhalant use increase as users get older?
- List the four stages of CNS depression from inhalant use. Provide at least one symptom for each stage.
- What is sudden sniffing death syndrome?
2nd edition
